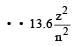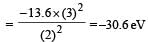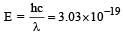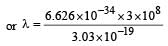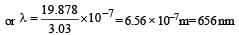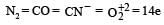31 Year NEET Previous Year Questions: Structure of Atom - 2 - NEET MCQ
30 Questions MCQ Test Chemistry Class 11 - 31 Year NEET Previous Year Questions: Structure of Atom - 2
The spectrum of He is expected to be similar to that[1988]
The number of spherical nodes in 3p orbitals are [1988]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If r is the radius of the first orbit, the radius of nth orbit of H-atom is given by [1988]
Which of the following statements do not form a part of Bohr ’s model of hydrogen atom ? [1989]
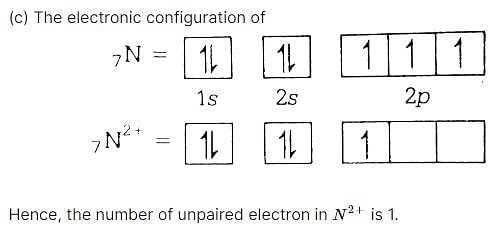
Number of unpaired electrons in N2+ is [1989]
The maximum number of electrons in a subshell is given by the expression [1989]
The total number of electrons that can be accommodated in all the orbitals having principal quantum number 2 and azimuthal quantum number 1 is [1990]
An ion has 18 electrons in the outermost shell, it is[1990]
In a given atom no two electrons can have the same values for all the four quantum numbers.This is called [1991]
For azimuthal quantum number ℓ = 3, the maximum number of electrons will be [1991]
The order of filling of electrons in the orbitals of an atom will be [1991]
The energy of an electron in then the Bohr orbit of hydrogen atom is [1992]
Which of the following species has four lone pairs of electrons?
If ionization potential for hydrogen atom is 13.6 eV, then ionization potential for He+ will be
For which one of the following sets of four quantium numbers, an electron will have the heighst energy? [1994]
Which of the following is never true for cathode rays ?[1994]
The electron was shown experimentally to have wave properties by [1994]
When an electron of charge e and mass m moves with a velocity v about the nuclear charge Ze is circular orbit of radius r, the potential energy of the electrons is given by [1994]
In the photo-electron emission, the energy of the emitted electron is [1994]
If electron has spin quantum number + 1/2 and a magnetic quantum number – 1, it cannot be present in [1994]
The radius of hydrogen atom in the ground state is 0.53 Å. The radius of Li2+ ion (atomic number = 3) in a similar state is [1995]
Uncertainty in position of an electron (mass = 9.1 × 10–28 g) moving with a velocity of 3 × 104 cm/s accurate upto 0.001% will be (use h/4π) in uncertainty expression where h = 6.626 ×10–27 erg-second) [1995]
The orbitals are called degenerate when [1996]
The momentum of a particle having a de Broglie wavelength of 10–17 metres is [1996] (Given h = 6.625 × 10–34 Js)
The ion that is isoelectronic with CO is [1997]
The Bohr orbit radius for the hydrogen atom (n = 1) is approximately 0.530 Å. The radius for the first excited state (n = 2) orbit is (in Å) [1998]
The position of both , an electron and a helium atom is known within 1.0 nm. Further the momentum of the electron is known within 5.0 × 10–26 kg ms–1. The minimum uncertainty in the measurement of the momentum of the helium atom is [1998]
According to Bohr ’s theory the energy required for an electron in the Li2+ ion to be emitted from n = 2 state is (given that the ground state ionization energy of hydrogen atom is 13.6 eV)
If the energy of a photon is given as : = 3.03 × 10–19 J then, the wavelength (λ) of the photon is :
Set of isoelectronic species is [2000]
|
127 videos|244 docs|87 tests
|


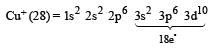
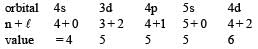
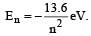
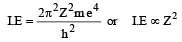
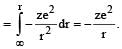
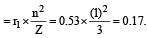
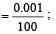
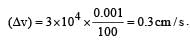
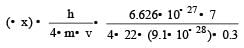
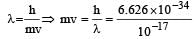 .
.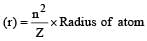
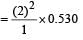
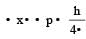 (which is constant)
(which is constant)