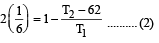31 Year NEET Previous Year Questions: Thermodynamics - 2 - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Previous Year Questions: Thermodynamics - 2
First law of thermodynamics is consequence of conservation of [1988]
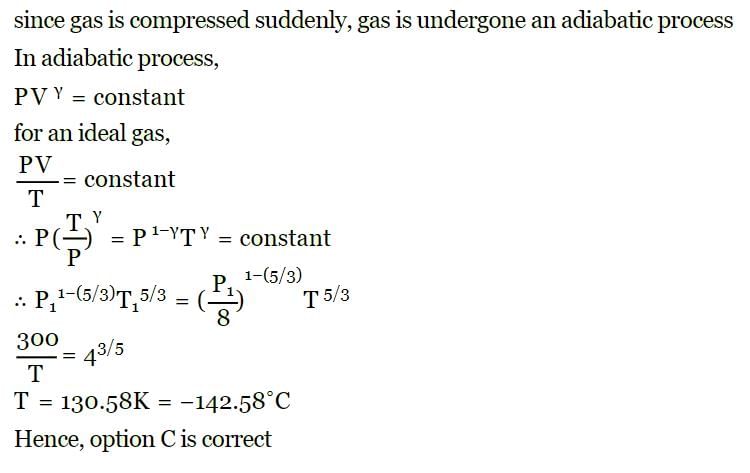
At 27° Ca gas is compressed suddenly such that its pressure becomes (1/8) of original pressure. Final temperature will be (γ = 5/3)
A thermodynamic process is shown in the figure.
The pressures and volumes corresponding to some points in the figure are
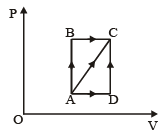
PA = 3 × 104 Pa
VA = 2 × 10-3 m3
PB = 8 × 104 Pa
VD = 5 × 10–3 m3.
In process AB, 600 J of heat is added to the system and in process BC, 200 J of heat is added to the system. The change in internal energy of the system in process AC would be [1991]
The pressures and volumes corresponding to some points in the figure are

VA = 2 × 10-3 m3
PB = 8 × 104 Pa
VD = 5 × 10–3 m3.
In process AB, 600 J of heat is added to the system and in process BC, 200 J of heat is added to the system. The change in internal energy of the system in process AC would be [1991]
For hydrogen gas, Cp – Cv = a and for oxygen gas, Cp – Cv = b, so the relation between a and b is given by [1991]
If for a gas, 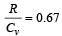 , the gas is made up of molecules which are [1992]
, the gas is made up of molecules which are [1992]
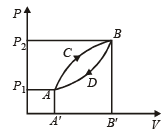
A thermodynamic system is taken from state A to B along ACB and is brought back to A along BDA as shown in the PV diagram. The net work done during the complete cycle is given by the area[1992]
An ideal gas A and a real gas B have their volumes increased from V to 2V under isothermal conditions. The increase in internal energy [1993]
110 joules of heat is added to a gaseous system, whose internal energy is 40J; then the amount of external work done is [1993]
Which of the following is not thermodynamical function ? [1993]
An ideal carnot engine, whose efficiency is 40% receives heat at 500 K. If its efficiency is 50%, then the intake temperature for the same exhaust temperature is [1995]
An ideal gas undergoing adiabatic change has the following pressure-temperature relationship [1996]
A diatomic gas initially at 18ºC is compressed adiabatically to one eighth of its original volume.The temperature after compression will be [1996]
A sample of gas expands from volume V1 to V2.The amount of work done by the gas is greatest, when the expansion is [1997]
The efficiency of a Carn ot engine operating between the temperatures of 100ºC and –23ºC will be
[1997]
If the ratio of specific heat of a gas at constant pressure to that at constant volume is γ, the change in internal energy of a mass of gas, when the volume changes from V to 2V at constant pressure P, is [1998]
We consider a thermodynamic system. If ΔU represents the increase in its internal energy and W the work done by the system, which of the following statements is true? [1998]
An ideal gas at 27ºC is compressed adiabatically to 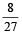 of its original volume. The rise in temperature is
of its original volume. The rise in temperature is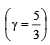 [1999]
[1999]
A reversible engine converts one-sixth of the heat input into work. When the temperature of the sink is reduced by 62ºC, the efficiency of the engine is doubled. The temperatures of the source and sink are [2000]
If γ be the ratio of specific heats of a perfect gas, the number of degrees of freedom of a molecule of the gas is [2000]
The temperature of source and sink of a heat engine are 127ºC and 27ºC respectively. An inventor claims its efficiency to be 26%, then:
A gas at 27ºC temperature and 30 atmospheric pressure is allowed to expand to the atmospheric pressure. If the volume becomes 10 times its initial volume, then the final temperature becomes
A Carnot engine whose efficiency is 50% has an exhaust temperature of 500 K. If the efficiency is to be 60% with the same intake temperature, the exhaust temperature must be (in K) [2002]
An ideal gas heat engine operates in a Carnot cycle between 227ºC and 127ºC. It absorbs 6 kcal at the higher temperature. The amount of heat (in kcal) converted into work is equal to
One mole of an ideal gas at an initial temperature of T K does 6R joules of work adiabatically. If the ratio of specific heats of this gas at constant pressure and at constant volume is 5/3, the final temperature of gas will be [2004]
Which of the following processes is reversible?[2005]
An ideal gas heat engine oper ates in Car not cycle between 227°C and 127°C. It absorbs 6 × 104 cals of heat at higher temperature. Amount of heat converted to work is [2005]
A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased so as to increase, its efficiency by 50% of original efficiency ? [2006]
The molar specific heat at constant pressure of an ideal gas is (7/2) R. The ratio of specific heat at constant pressure to that at constant volume is
An engine has an efficiency of 1/6. When the temperature of sink is reduced by 62°C, its efficiency is doubled. Temperature of the source is
If Q, E and W denote respectively the heat added, change in internal energy and the work done in a closed cyclic process, then: [2008]



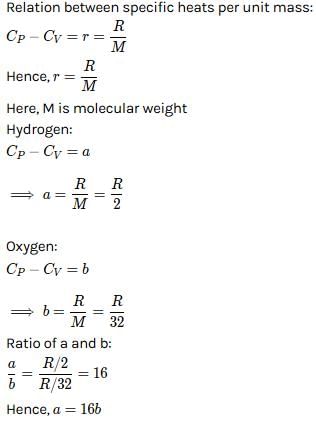
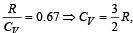 hence gas is monoatomic.
hence gas is monoatomic.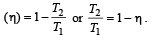
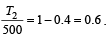
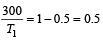
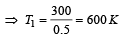
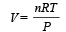 ....(2)
....(2)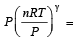 constant
constant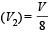
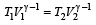
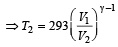
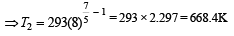
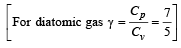
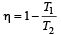
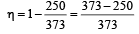
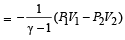
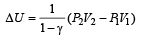
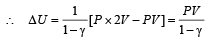
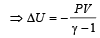
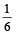 which increases to
which increases to 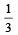 when the sinktemperature reduces by 62º C.
when the sinktemperature reduces by 62º C. when T2 = sink temperature T1 = source temperature
when T2 = sink temperature T1 = source temperature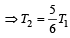
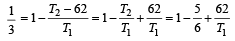
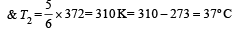
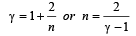
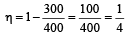
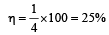
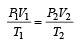
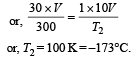
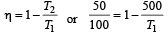
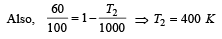
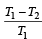
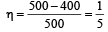
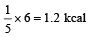
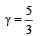
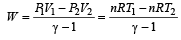
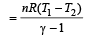
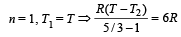
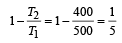
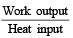
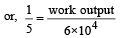
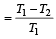
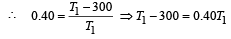
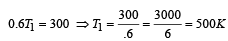
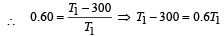
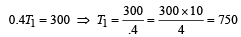
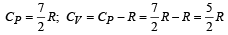
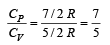
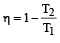
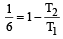 .......... (1)
.......... (1)