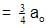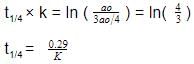AWES PGT Chemistry Mock Test - 4 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2024 - AWES PGT Chemistry Mock Test - 4
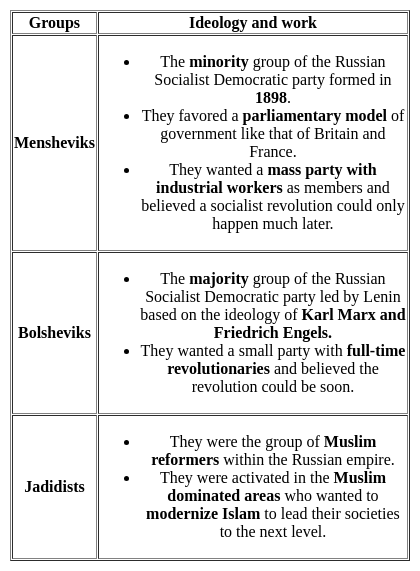
Name the majority group of the Russian Social Democratic Workers Party led by Lenin?
Recently, which institute hosted the International Chess Federation (FIDE) rated chess tournament?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The First Cotton Textile Mill was established in India at
Which of the following statements is NOT correct in the context of teaching area- measurement of plane figures?
‘Choice of challenge’ is a characteristic of which of the following?
How does NEP 2020 address the issue of gender-based discrimination in education?
Which of the following elements belongs to the same group as the element having configuration 1s2 2s2 2p1
A gas absorbs 200 J of heat and expands by 500 cm3 against a constant pressure of 2 x 105 Nm-2. Change in internal energy is
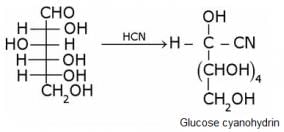
What does the following reaction shows about the structure of glucose?
Identify the correct statements with reference to the given reaction
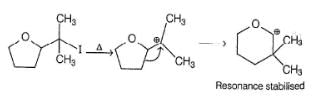
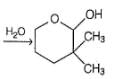
Which is the most likely product when the following iodide is heated with water?
The enthalpy change when 1 mole of it dissolves in a specified amount of solvent is known as enthalpy of
Passing carbon dioxide through slaked lime gives:
t1/4 can be taken as the time taken for the concentration of a reactant to drop to 3/4 of its initial value. If the rate constant for a first order reaction is K, t1/4 can be written as –
[AIEEE-2005]
The standard reduction potential of a silver chloride electrode is 0.2 V and that of a silver electrode is 0.79 V. The maximum amount of AgCl that can dissolve in 106 L of a 0.1 M AgNO3 solution is
Which of the following amine will form stable diazonium salt at 273-283 K ?
Mean bond enthalpy of different bonds are given
Out of the given pairs, which compound is more stable than the other?
Consider the following elimination reaction,
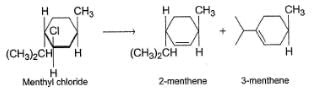
When reaction is carried out with C2H5ONain ethanol, 2-menthene is the major product while 3-menthene is the major product if reaction is substrate is heated in ethanol only. It is due to
|
12 docs|30 tests
|
|
12 docs|30 tests
|