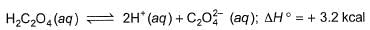AWES PGT Chemistry Mock Test - 6 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2024 - AWES PGT Chemistry Mock Test - 6
Which of the following is not a part of universal design for learning guidelines?
Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, l = 1 and 2 are, respectively [AIEEE- 2004]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the electrorefining of metals, impure metal is
C—Cl bond in (vinyl chloride) is stabilised in the same way as in
Which physical quantity remain unaffected when a reversible reaction is carried out in presence of a catalyst
Thermal decomposition of a compound is of first order. If 50% of a sample of a compound is decomposed in 120 min, the time taken for 90% completion is
Which of the following correctly describe the relative nucleophilicities of methoxide and tertiary butoxide ion?
Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, = 1 and 2 are, respectively:
[Ti (H2O)6]3+ absorbs green and yellow region part of visible light. Then the transmitted colour of the compound is
The conditions favourable for the reaction :
2SO2(g)+O2(g) 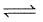 2SO3(g) ; ΔH° = -198 kJ are :
2SO3(g) ; ΔH° = -198 kJ are :
In the electrolysis of aqueous sodium chloride solution, two types of reactions can take place at anode :
I. 2Cl- (aq) → Cl2(g) +2e-
II. 2H2O(l)g → O2 (g) + 4H+(aq) + 4e-
Select the correct statement(s) about these.
For the reaction,
2SO2 (g) + O2 (g)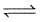 2SO3 (g) + 188.3 KJ
2SO3 (g) + 188.3 KJ
the number of moles of SO3 formed is increased if
The metabolism of hormones in human body is an example of
Which of the following, do you think are the synthetic resins present in removal of permanent hardness?
90% of hydrogen peroxide is used as fuel in ______________
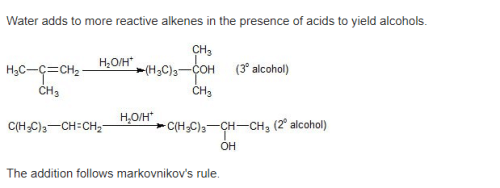
Acid catalyzed hydration of alkenes except ethene leads to the formation of –
[AIEEE-2005]
In the following reactions except in one, oxygen is the reducing agent. Exceptional reaction is
Pyrolusite in MnO2 is used to prepare KMnO4. Steps are
Here, I and II are
Newland's Law of Octaves suggested that elements exhibited similar properties at regular intervals. However, this law failed to accommodate the discovery of:
Among the following elements element with highest ionisation enthalpy is:
Gadolinium belongs to 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
Which of the following molecule doesn’t have a lone pair?
The process in which colloid is placed inside a bag of semi permeable membrane like cellophane or parchment paper which permits ions and not colloids to pass through, is called as:
Out of the following, maximum covalent nature is in
Mole fraction of C3H5(OH)3 in a solution of 36 gm of water and 46 gm of glycerine is
|
12 docs|30 tests
|
|
12 docs|30 tests
|