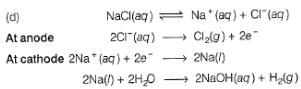AWES PGT Chemistry Mock Test - 7 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2024 - AWES PGT Chemistry Mock Test - 7
What’s the atomic number of the element Copernicium?
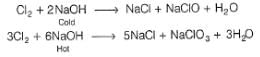
What product are formed during the electrolysis of a concentrated aqueous solution of sodium chloride using an electrolytic cell in which electrodes are separated by a porous pot?
I. Cl2(g)
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq)
Select the correct choice.
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C?
The substance that gets adsorbed on the surface of the solid is called?
Reaction of granulated zinc with dil HCl results in formation of:
Which of the following is prepared by cyanamide process?
One of the following rubbers is used in making oil seals, tank lining, etc.
Direction (Q. Nos. 7 - 10) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Predict product(s) in the following reaction.
Passage III
A solution containing 0.684 g of cane sugar (C12H22011) in 100 g water freezes at - 0.037° C. A solution containing 0.585 g of NaCI in 100 g water freezes at - 0.342° C.
Q.
Apparent molecular weight of NaCI is
(Yellow ppt) T X
Y. (Yellow ppt) + Z (pungent smellinggas)
If X gives green flame test. Then, X is :
The primary difference between the modern periodic table and Mendeleev's periodic table is:
In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3 . Taking kƒ for water as 1.85, the freezing point of the solution will be nearest to _
[AIEEE-2003]
Predict the product in nucleophilic substitution reaction.
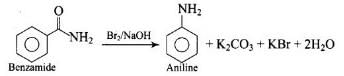
Hoffmann Bromamide Degradation reaction is shown by __________.
A __________ overlap doesn’t result in the formation of a bond.
Statement Type
Direction (Q. Nos. 16 and 17) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Consider the following two bromides I and II, undergoing solvolysis reaction in boiling ethanol :
Statement I : I is less reactive than II in the given solvolysis reaction.
Statement II : Resonance stabilisation available with the intermediate formed from II is the important driving force.
The main product formed by treating an alkyl or benzyl halide with excess ammonia
Which of the following is the best example of shape selective catalysis?
Which option is incorrect among the following for the given property?
A solution of NH4CI is prepared by dissolving 95 g of NH4CI in 200 g of H2O at 60° C. What is mass per cent when the solution is cooled to 20° C based on this
How many grams of water would you add to 1.38 moles of CH3OH in 1 kg water to reduce the molality to 1.00 molal CH3OH(aq) ?
Addition of bromine on propene in the presence of brine yields a mixture of
Passage
Phosphorus was discovered by Brand (1669), Scheele isolated from bone ash and Lavoisier proved its elemental nature (1777). The principal minerals are phosphate rock, fluoroacetate, and chloroacetate. Phosphorus is prepared by the direct reduction of phosphorite by carbon in the presence of silica. It exists in different allotropic forms such as yellow or white, red, a-black,f3-black, etc. White P is most reactive, poisonous, glows in dark, and readily catches fire due to unstable discrete P4 molecules. Red P is inert, non-poisonous, does not glow, etc., due to its polymeric structure. a-black, f3 -black allotropes are also chemically inert, do not ignite at normal temperature. It has a layer structure like graphite and acts as a conductor.
Q. The allotrope of phosphorus that has layer lattice-like graphite is:
Milk turns sour at 40°C three times faster than it does at 0°C, this shows that activation energy of souring of milk (in cal) is
|
12 docs|30 tests
|
|
12 docs|30 tests
|