Chemical Bonding & Periodicity - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Chemical Bonding & Periodicity
The number of species having 3c-2e- bond among following is/are____
Al2(CH3)6, AI2(CH3)4CI2,(MgH2)2, AI(BH4)3, BeCI2(in solid)
Al2(CH3)6, AI2(CH3)4CI2,(MgH2)2, AI(BH4)3, BeCI2(in solid)
The percentage s-character in lone pair in PH3 molecule having bond angle 92º is _____________
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Correct order of ionization energy among following is/are
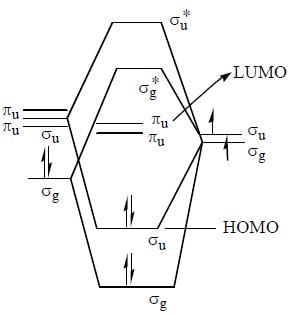
(I) N2 > N > O (II) N > O > O2 (III) N2 > O2 > NO (IV) N2 > C2 > O2
(I) N2 > N > O (II) N > O > O2 (III) N2 > O2 > NO (IV) N2 > C2 > O2
Correct set of point group for BrF5, 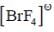 and [BrF6]+ respectively are-
and [BrF6]+ respectively are-
The maximum number of atoms that may lie in the same plane in molecule P(CH3)3(CF3)2 is _______
Number of molecule that show fluxional behavior among. CH4,CF4,PCI5,PF5,BrF5,SF6,XeO3 is _________
The number of anions having perfect Oh geometry among
[SbCI6]3-, [SeCI6]2-,[TeBr6]2-,[BrF6]-,[IF6]-,[CIF6]-,[SeF6]2- is/are__________
Regarding following compounds A,B,C and D
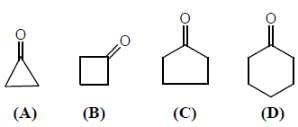
Correct statement(s) is/are

The ionisation potential of hydrogen atom is 13.6 eV. The first ionisation potential of a sodium atom, assuming that the energy of its outer electron can be represented by a H-atom like model with an effective nuclear charge of 1.84, is
In salt [Et4N]2[InCI5] and [NH3(CH2)5(NH3)][TiCI5], structures of anions respectively are
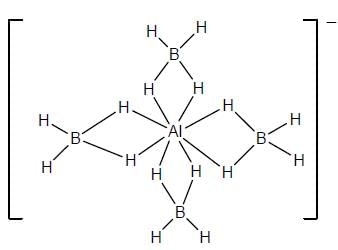
Select the correct statement regarding [Ph3MeP][AI(BH4)4]
(I) At 298 anion show one signal in 1H NMR
(II) At 298 anion show two signal in 1H NMR
(III) At 298 a quintet is observed in 11B NMR
(IV) At 298 a triplet of triplet is observed in 11B NMR
The ground state terms for H+2 and No respectively.
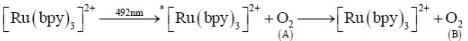
The final dioxygen (B) undergo Diels Alder reaction unlike normal dioxygen(A). The value of spin multiplicity which represent ground state term of dioxygen (B) is __________
|
18 docs|37 tests
|
|
18 docs|37 tests
|


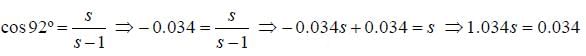
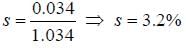
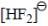 molecule
molecule 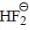
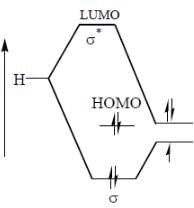
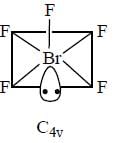
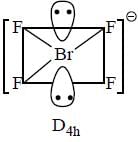
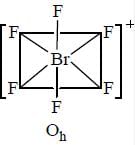
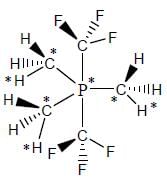
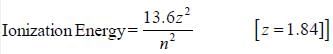
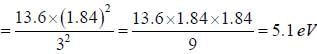
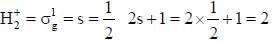
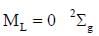
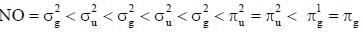
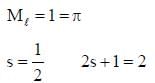
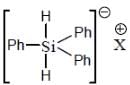
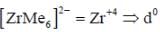 perfect trigonal prismatic geometry
perfect trigonal prismatic geometry
















