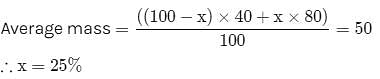Chemistry: Topic-wise Test- 1 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - Chemistry: Topic-wise Test- 1
The vapour density of a mixture of gas A (Molecular mass = 40) and gas B (Molecular mass = 80) is 25.Then mole % of gas B in the mixture would be
Atomic mass of Cl is 35.5 g. Calculate the mass of 4.50 moles of chlorine gas, Cl2.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Atomic mass of bromine is 80 g. The mass of four moles of molecular bromine (Br2) is:
Modern periodic table is based on atomic no. experiments which proved importance of at no. was -
Atomic no. is the base of -
(i) Lother meyer curve
(ii) Newland octave rule
(iii) Modern periodic table
(iv) Doeberiener triad rule
(v) Long form of periodic table
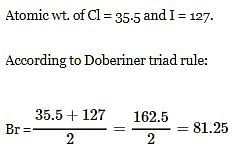
Atomic wt. or Cl = 35.5 and of I = 127. According to doeberiner triad rule, At. wt. of Br will be -
Which of the following molecules are expected to exhibit intermolecular H-bonding
I. Acetic acid
II. o-nitrophenol
III. m-nitrophenol
IV. o-boric acid
A : tetracyanomethane B : Carbondioxide
C : Benzene D : 1, 3-buta-di-ene
Ratio of σ and π bond is in order:
Which of the following models best describes the bonding within a layer of the graphite structure ?
In , the shape is square planer. The number of bond pair-lone pair repulsion at 90° are :
In which of the following molecules / ions all the bonds are not of equal length.
The critical temperature of water is higher than that of O2 because the H2O molecules has :
Among these canonical structures, the correct order of stability is
For phenol which ofthe following resonating structure is the most stable ?
Among these three canonical structures (through more are possible) what would be their relative contribution in the hybrid
The most stable resonating structure of following compound is
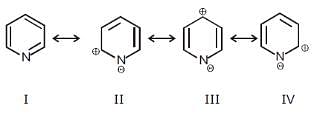
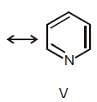
Among these canonical structures of pyridiine, the correct order of stability is
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which of the following reaction would not give atleast one aldehyde product?
Which set of reagent(s) in correct order would accomplish the following transformation?

What is the final major product of the following reaction?
Hydrogenation of alkenes can be carried out in the presence of
What is the major addition product in the reaction given below?
Direction (Q. Nos. 12 and13) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Caryophyllene (C15H24) contain a six membered ring and on ozonolysis gives following product.
Q. The structure of caryophyllene is
Caryophyllene (C15H24) contain a six membered ring and on ozonolysis gives following product.
Q. If caryophyllene is treated with 1.0 mole of HCI, a ring closure reaction takes place to form monochloride. What is the most likely product of this reaction?
Direction (Q . No. 14) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q. Match the alkenes from Column I with the stereochemistry of addition product(s) obtained with Br2/CCI4 in presence of FeBr3.
Ethanol has a higher boiling point than dimethyl ether though they have the same molecular weight. This is due to :
Arrange the following in order of decreasing boiling point :
(I) n-Butane (II) n-Butanol (III) n-Butyl chloride (IV) Isobutane
Which of the following compounds would have significant intermolecular hydrogen bonding ?
HF, CH3OH, N2O4, CH4
For H2O2, H2S, H2O and HF , the correct order of decreasing extent of hydrogen bonding is :
|
1 videos|26 docs|111 tests
|
|
1 videos|26 docs|111 tests
|