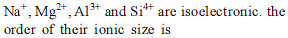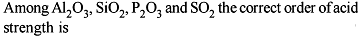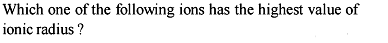Classification of Elements and Periodicity in Properties - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Classification of Elements and Periodicity in Properties - 1
Which is the correct order of ionic sizes (At. No. : Ce = 58, Sn = 50, Yb = 70 and Lu = 71) [AIEEE-2002]
The reduction in atomic size with increase in atomic number is a characteristic of elements of - [AIEEE-2003]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
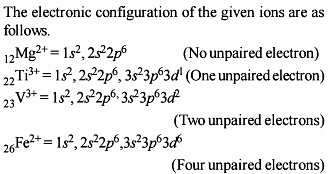
The atomic numbers of vanadium (V). Chromium (Cr), manganese (Mn) and iron (Fe) respectively 23, 24, 25 and 26. Which one of these may be expected to have the higher second ionization enthalpy ?
[AIEEE-2003]
Which one of the following sets of ions represents the collection of isoelectronic species ? [AIEEE-2004]
lanthanoid contraction is caused due to - [AIEEE-2006]
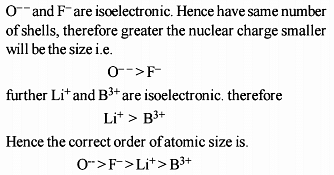
The increasing order of the ionic radii of the given isoelectronic species is:
Choose the correct order of the following:
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|