DSSSB PGT Chemistry Mock Test - 4 - DSSSB TGT/PGT/PRT MCQ
30 Questions MCQ Test DSSSB PGT Mock Test Series 2024 - DSSSB PGT Chemistry Mock Test - 4
How many times are the hands of a clock at right angles in a day?
Directions to Solve
In each of the following questions find out the alternative which will replace the question mark.
Question -
Melt : Liquid :: Freeze : ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Direction: Read the following passages carefully and answer the question that follows.
The ancient Harappan Civilization emerged, flourished and collapsed under a steadily weakening monsoon, according to new research findings, that scientists say, provide the strongest evidence yet to link its risk and fall to changing climate. A team of scientists has combined multiple sets of date to show that weakening monsoon and reduced river water initially stimulated intensive agriculture and urbanisation, but later precipitated the decline and collapse of the subcontinent's earliest cities. The scientist said their research also suggests that a larger river, summed to be the mythical Saraswati, which once watered the Harappan Civilization's heartland between the suggests it was a glacier fed river with origins in the Himalyas. The findings appear today in the US Journal Proceedings of the National Academy of Science
Q. What was the controversy in the passage?
Direction: In many of the process control applications, the purpose of control system is to keep the output (controlled variable) almost constant in spite of changes in load. Mostly in continuous processes the set point remains constant for longer time. Such an operation is called 'Regulator Operation'. The set point generated, and the actual values from sensors are given to a controller. The controller compares both the signals, generates error signal which is utilized to generate a final signal as controller output. The controller output is finally utilized to physically change the values of manipulated variable to achieve stability. The above action is achieved with the help of final control elements. They are operatable either with electrical, pneumatic or with hydraulic signals. The system that serves good for servo operation will generally not be the best for regulator operation. Large capacity or inertia helps to minimize error here, whereas it makes the system sluggish in case of servo operation.
Q. What is the purpose of the control system?
By selling a cap for ₹ 29.75, a man gains 6.25%. What will be the CP of the cap?
A fires 5 shots to B's 3 but A kills only once in 3 shots while B kills once in 2 shots. When B has missed 27 times, A has killed:
नीचे दिए गए वाक्य का प्रकार बताइये।
मुसीबत आ जाए तो भागना उचित नहीं।
In scientific notation for such numbers, any number can be represented in the form N × 10n where
Hydrogen bonding occurs in which type of crystalline solids?
Dalton's atomic theory could not explain one of the following
Calculate the wavelength (in nanometer) associated with a proton moving at 1.0×103ms-1 (Mass of proton = 1.67×10-27kg and h = 6.63×10-34Js)
Which of the following metal solution cannot be prepared by Bredig’s arc method?
A solution was made by dissolving 2 g of a solute in 100 g of acetone. The solution boiled at 56.95° C. The boiling point of pure acetone is 55.95° C, and the Kb =1.71°C/m. What is the molecular weight of the solute?
Which of the following gaseous molecule is monoatomic?
Sodium pentacyanonitrosylferrate(II) is also called?
Requirement of macronutrient per acre of the land is
Which of the following statements is correct about Enzymes?
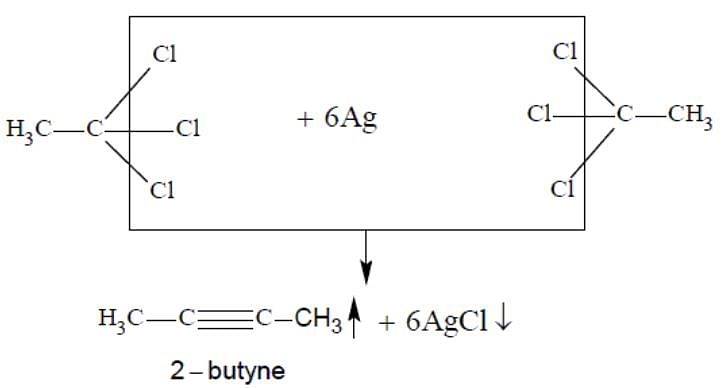
The major organic compound formed by the reaction of 1,1,1-trichloroethane with silver powder is: