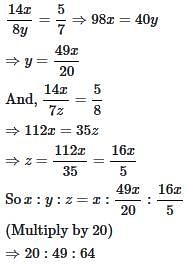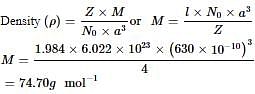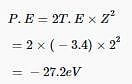DSSSB PGT Chemistry Mock Test - 5 - DSSSB TGT/PGT/PRT MCQ
30 Questions MCQ Test DSSSB PGT Mock Test Series 2024 - DSSSB PGT Chemistry Mock Test - 5
If the 1st of March of a year is a Wednesday, then which of the following months of the same year will start with the same day?
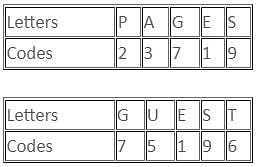
In a certain coding system, 'PAGES' is coded as 23719 and 'GUEST' is coded as 75196. How will you code 'STAGE' in this coding system?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Cardiac muscle is one of the three major types of muscles, the others being skeletal and smooth muscles. It is found in the walls and histological foundation of the heart. Which one of the following statements is not related to the cardiac muscles?
Three partners shared the profit in a business in the ratio 5 : 7 : 8. They had partnered for 14 months, 8 months and 7 months respectively. What was the ratio of their investments?
नीचे दिए गए पद इक प्रत्यय लगाने से बने है, इनमें से कौन सा पद गलत है?
निर्देशः नीचे दिए गए अपठित गद्यांश को ध्यानपूर्वक पढ़ें एवं उस पर आधारित प्रश्न के उत्तर दें।
शिक्षा जीवन के सर्वांगीण विकास हेतु अनिवार्य है। शिक्षा के बिना मनुष्य विवेकशील और शिष्ट नहीं बन सकता। विवेक से मनुष्य में सही और गलत का चयन करने की क्षमता उत्पन्न होती है। विवेक से ही मनुष्य के भीतर उसके चहुँ ओर नित्य प्रति होते घटनाक्रमों के प्रति एक छिद्रान्वेषी दृष्टिकोण उत्पन्न होता है। शिक्षा ही मानव को मानव के प्रति मानवीय भावनाओं से पोषित करती है। शिक्षा से मनुष्य अपने परिवेश के प्रति जाग्रत होकर कर्तव्यविमुख हो जाता है। 'स्व' से 'पर' की ओर अग्रसर होने लगता है। निर्बल की सहायता करना, दुखियों के दु:ख दूर करने का प्रयास करना, दूसरों के दुःख से दु:खी हो जाना और दूसरों के सुख से स्वयं सुख का अनुभव करना जैसी बातें एक शिक्षित मानव में सरलता से देखने को मिल जाती हैं। इतिहास, साहित्य, राजनीतिशास्त्र, समाजशास्त्र, दर्शनशास्त्र इत्यादि पढ़कर विद्यार्थी विद्वान् ही नहीं बनता वरन् उसमें एक विशिष्ट जीवन दृष्टि, रचनात्मकता और परिपक्वता का सृजन भी होता है। शिक्षित सामाजिक परिवेश में व्यक्ति अशिक्षित सामाजिक परिवेश की तुलना में सदैव ही उच्च स्तर पर जीवन यापन करता है।
परन्तु आज शिक्षा का अर्थ बदल रहा है। शिक्षा भौतिक आकांक्षा की साध्य बनती जा रही है। व्यावसायिक शिक्षा के अन्धानुकरण में छात्र सैद्धान्तिक शिक्षा से दूर होते जा रहे हैं। रूस की क्रान्ति, फ्रांस की क्रान्ति, अमेरिकी क्रान्ति, समाजवाद, पूँजीवाद, राजनीतिक व्यवस्था, सांस्कृतिक मूल्यों आदि की सामान्य जानकारी भी व्यावसायिक शिक्षा ग्रहण करने वाले छात्रों को नहीं है। यह शिक्षा का विशुद्ध रोजगारकरण है। शिक्षा के प्रति इस प्रकार का संकुचित दृष्टिकोण अपनाकर विवेकशील नांगरिकों का निर्माण नहीं किया जा सकता। भारत जैसे विकासशील देश में शिक्षा रोजगार का साधन न होकर साध्य हो गई है। इस कुप्रवृत्ति पर अंकुश लगाना अनिवार्य है। जहाँ मानविकी के छात्रों को पत्रकारिता, साहित्य-सृजन, विज्ञापन, जनसम्पर्क इत्यादि कोर्स भी कराए जाने चाहिए ताकि उन्हें रोजगार के लिए न भटकना पड़े, वहीं व्यावसायिक कोर्स करने वाले छात्रों को मानविकी के विषय; जैसे-इतिहास, साहित्य, राजनीतिशास्त्र व दर्शन आदि का थोड़ा बहुत अध्ययन अवश्य कराना चाहिए ताकि समाज को विवेकशील नागरिक प्राप्त होते रहें, तभी समाज में सन्तुलन बना रहेगा।
Q. शिक्षा से मनुष्य स्व' से 'पर' की ओर अभिगमन करने लगता है, क्यों?
A substance forms face centered cubic crystals. Its density is 1.984 g/cm3 and the length of the edge of the unit cell is 630 pm. Calculate the molar mass in g/mol?
Radii of A+ and that of X- and Y- have been given as
A+= 1.00 pm
X- = 1.00 pm
Y- = 2.00 pm
Thus, ratio of volumes of A X and A Y unit cells is
The potential energy of an electron in the second Bohr's orbit of the he±
In Lyman series, shortest wavelength of H-atom appears at x m, then longest wavelength in Balmer series of He+ appear at
A solution of copper(II) sulphate (VI) is electrolysed between copper electrodes by a currrent of 10.0 A for exactly 9650 s.Which remains unchanged?
Covalent nature of NaF, Na2O and Na3N in the increasing order is
Given for HCI and HI
Q. Ratio of fraction of electric charge existing on each atom in HCI and HI is
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:
The modern periodic table is divided into how many blocks?
What is the most efficient method to get water with zero degrees hardness?
Which of the correct statement for the given acids?
Which of the following statement is incorrect ?
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data etc. Two questions related to the paragraph have been given. Each has only one correct answer among the four given options (a) ,(b), (c) and (d).
Passage
Consider the following isomers of [Co(NH3)4Br2]+. The black sphere represents Co, grey sphere represents NH3 and unshaded sphere represents Br.
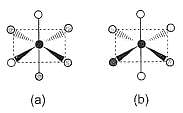

Q.
The oxidation state and coordination number of cobalt in the complex [Co(NH3)4Br2]+ are
Identify the pair of enantiomers amongst the given pairs:
The reaction of SOCI2 on alkanols to form alkyl chlorides gives good yields because
What is the major product of the given reaction ?
Chloramphenicol is used to treat typhoid, dysentery, acute fever, meningitis etc., because it is