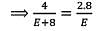DSSSB PGT Chemistry Mock Test - 8 - DSSSB TGT/PGT/PRT MCQ
30 Questions MCQ Test DSSSB PGT Mock Test Series 2024 - DSSSB PGT Chemistry Mock Test - 8
How many days will there be from 23rd January, 2011 to 31st July, 2013 (both days included)?
Two clocks are set correctly at 10 a.m. on Friday. The first clock gains 2 minutes per hour, which is twice as much as gained by the second clock. What time will the second clock register when the correct time is 2 p.m. on the following Monday?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A clock gains 2 minutes in an hour and an other clock loses 4 minutes in an hour. If both these clocks were set at 8 a.m, what will be the time in the first clock, if the second clock shows 10 p.m?
Direction: Read the following passage carefully and answer the questions. Your answer to these questions should be based on passage only.
A stout old lady was walking with her basket down the middle of a street in retrograde to the great confusion of the traffic and no small peril to herself. It was pointed out to her that the pavement was the place for foot-passengers, but she replied, 'I m going to walk where I like. We have got liberty now.' It did not occur to the dear lady that if liberty entitled the foot-passenger to walk down the middle of the the road it also entitled the taxi-drive on the pavement, and that the end of such liberty would be universal chaos. Everything would be getting in everybody else's way and nobody would get anywhere. Individual liberty would have become social anarchy
Q. The lady refused to move from the middle of the street because?
Direction: In 1900, to a shipping clerk---Alexander Fleming---a career in science seemed like a distant dream. Alexander the youngest son of a Scottish farmer from Ayrshire was born on 6, August, 1881. He was able to complete High school but then his family funds ran out. At sixteen he took a job as a shipping clerk and stayed there for four years. In 1901. Alexander came across a small legacy which enabled him to continue his education, and, on the advice of one of his brothers who was a doctor, he chose to prepare for a career in medicine. Alexander did unusually well in medical school along with rifle shooting, swimming, water polo and painting. After his graduation, his teacher Prof. Wright asked him to join him in bacteriological research, which he readily agreed.
Q. What problem was faced by Alexander that nipped his completion of High School ?
Directions to Solve:
Choose the correct alternative that will continue the same pattern and replace the question mark in the given series.
Question: 1, 2, 3, 6, 9, 18, ?, 54
A sum of Rs. 600 amounts to Rs. 720 in 4 years at Simple Interest. What will it amount to if the rate of interest is increased by 2%?
When a positive number n is divided by 7 leaves the remainder 2, when 3n is divided by the same number, then the remainder is
P, Q, R enter into a partnership. P initially invests 25 lakh & adds another 10 lakhs after one year. Q initially invests 35 lakh & withdrawal 10 lakh after 2 years and R invests Rs 30 Lakhs . In what ratio should the profit be divided at the end of 3 years?
A police saw a Thief at a distance of 2km. When Police started chasing him Thief also started running. If the ratio of Speeds of Police to Thief is 5:4. Then thief was caught at a certain distance then how many Kms did police run to catch the Thief?
In an experiment, 4 g of M2Ox oxide was reduced to 2.8g of the metal.Tf the atomic mass of the metal is 56 g mol-1, the number of O atoms in the oxide is
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Potassium crystallises in a bcc lattice as shown
Q.
Distance between the two nearest neighbours and between next nearest neighbour respectively, is
Which of the following sets of quantum numbers is correct for an electron in 4f orbital ? [AIEEE- 2004]
Which one of the following sets of ions represents the collection of isoelectronic species ? [AIEEE- 2004]
lf En = total energy, Kn = kinetic energy, Vn = potential energy and rn = radius of the nth orbit, then based on Bohr’s theory, match the parameter in Column I with the values in Column II.
Direction (Q. Nos. 11-14) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Passage I
Solid ammonium chloride is in equilibrium with ammonia and hydrogen chloride gases
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.
Q. Partial pressure of NH3(g) or HCI (g) at equilibrium is
The equilibrium constant for the reaction
CO(g) + H2O(g) 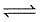 CO2(g) + H2(g) is 3 at 500 K. In a 2 litre vessel 60 gm of water gas [equimolar mixture of CO(g) and H2(g)] and 90 gm of steam is initially taken.
CO2(g) + H2(g) is 3 at 500 K. In a 2 litre vessel 60 gm of water gas [equimolar mixture of CO(g) and H2(g)] and 90 gm of steam is initially taken.
What is the equilibrium concentration of H2(g) at equilibrium (mole/L) ?
pH of saturated solution of silver salt of monobasic acid HA is found to be 9.
Find the Ksp of sparingly soluble salt Ag A(s).
Given : Ka(HA) = 10-10
The solubility of Mg(OH)2 is x mole/ltr. then its solubility product is -
[AIEEE-2002]
Decomposition of H2O2 was studied by titration against KMnO4 solution. It was found that 0.4 mol of H2O2 was reduced to 0.2 mol in 20 min and to 0.1 mol in 40 min and to 0.05 mol after 1 hr, the order of reaction must be
In the equation Kt = log C0 – log Ct, the curve between t and log Ct is -
[AIEEE-2002]
Select the correct statement if -
E°Mg2+/Mg = - 2.4V, E°Sn4+/Sn2+ = 0.1 V, E°MnO4-,H+/Mn2+ = 1.5 V, E° I2/I- = 0.5 V Here,
What is the value of pKb (CH3COOH-) if λm∞ = 390 & λm = 7.8 for 0.04 of a CH3COOH at 25°C


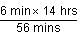 = 90 minutes.
= 90 minutes.