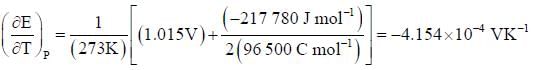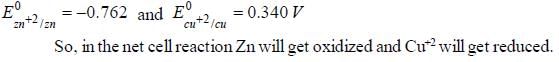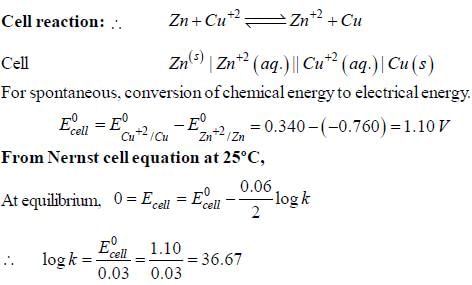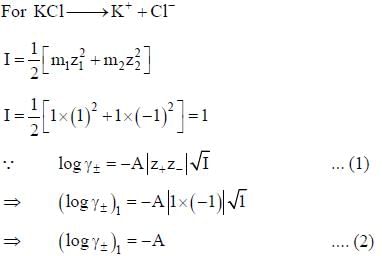Electrochemistry - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Electrochemistry
Equivalent conductance of saturated BaSO4 is 400 ohm-1cm2 equivalemt-1 and specific conductance is 8 x 10-5 ohm-1cm-1. Hence, ksp of BaSO4 is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
For a cell reaction, E (at 25oC) = 1.26V, n = 2 and ΔS = -96.5JK-1mol-1. E at 85oC by assuming ΔS to be independent of temperature is _____ (V). [Given F = 96500 Cmol-1] (Round off to two decimal places).
Given for the reaciton,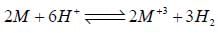 for which ΔHo = -3.0 kcal. The molar entropies are 6.5 cal/K for M, -22.2 cal/K for M+3, 31.2 cal/k for H2 and -10.0cal/K for H+.
for which ΔHo = -3.0 kcal. The molar entropies are 6.5 cal/K for M, -22.2 cal/K for M+3, 31.2 cal/k for H2 and -10.0cal/K for H+.
The value of Eo for the half cell, 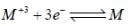 is ______[Round off to two decimal places]
is ______[Round off to two decimal places]
The resistance of decinormal solution of a salt occupying a volume between two platinum electrodes 1.80 cm apart and 5.4 cm2 area was found to be 32 ohm. The value of 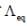 is
is
A current of 0.5A is passed through molten AlCl3 for 40 min. The mass of the aluminum deposited at the cathode is (Al = 27)
At 180C the mobilities of NH+4 and ClO-4 ions are 6.6 x 10-4 and 5.7 x 10-4 cm2 volt-1s-1 at infinite dilution. The value of equivalent conductance of ammonium perchlorate solution at infinite dilution
For the reaction,
H2(g)(1atm) + 2AgCL(s) = 2Ag(s) + 2H+(aq)(0.1M)+2Cl-(aq)(0.1M)
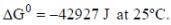 The emf of the cell is _____volt.(Round off to two decimal placs)
The emf of the cell is _____volt.(Round off to two decimal placs)
The variation of equivalent conductance of strong electrolyte with 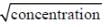 is correctly shown in the figure
is correctly shown in the figure
According to Debye Huckel limiting law the mean activity coefficient of 0.5 x 10-4 mol / kg CaCl2 is at 25oC
Given that, E0(Fe3+,Fe) = -0.04V and E0(Fe2+,Fe) = -0.44V, the value of E0(Fe3+,Fe2+) is _______ (in V). (round off to two decimal places).
The solubility product of AgBr(s) is 5 x 10-13 at 298k. If the standard reduction potential of the half cell 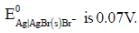 The standard reduction potential
The standard reduction potential 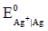 (in volts) is _____ (round off to two decimal places).
(in volts) is _____ (round off to two decimal places).
The concentration of a MgSO4 solution having the same ionic strength as that of a 0.1M Na2SO4 solution is ____(Round off to three decimal places)
The temperature coefficient of the cell is 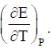 Choose the incorrect statement(S)
Choose the incorrect statement(S)
The standard electrode potential Eo at a fixed temperature and in a given medium is dependent on
At 273K, the calorimetric determination of ΔH for the reaction,
Zn(s) + 2AgCl(s) → ZnCl2(aq) + Ag(s)
yielded -217.78 kJ mol-1, while the emf of the corresponding cell was 1.015 V. The 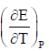 of the cell is
of the cell is
The Zn2+|Zn half cell (Eθ = -0.762V) is connected to a Cu2+ | Cu halff cell (Eo = 0.340V). The value of log10K, where K is the equilibrium constant is [Given : (2.303 RT/F) = 0.06]
An electrochemical cell consists of two half-cell reactions

The mass of copper (in grams) dissolved on passing 0.5 A current for 1 hour is
[Given: atoms mass of Cu is 63.6; F = 96500C mol-1]
According to the Dubye-Huckel limiting law, if the concendration of the dilute aqueous solution of KCI is increased 4-fold, the value of log 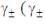 is the molal mean ionic activity coefficient) will be
is the molal mean ionic activity coefficient) will be
|
18 docs|37 tests
|
|
18 docs|37 tests
|


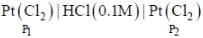 cell reaction will be spontaneous if:
cell reaction will be spontaneous if: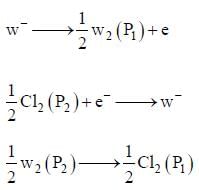
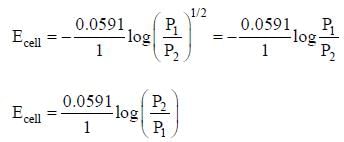
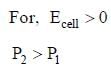
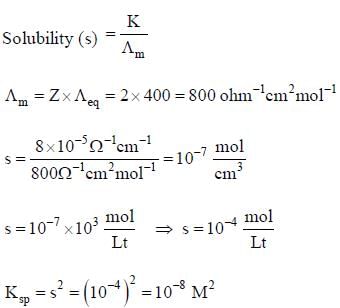
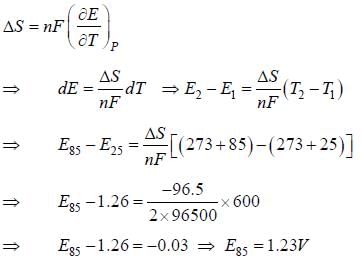
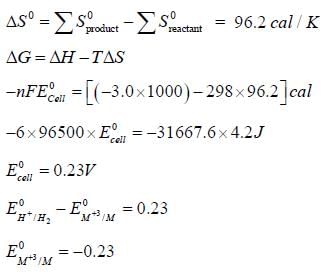
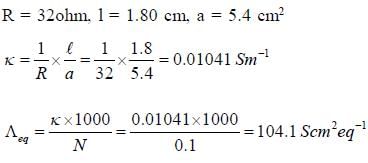
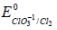
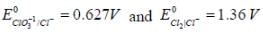
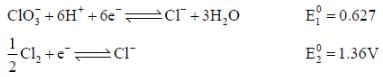
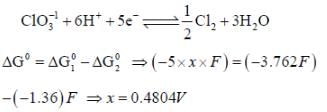
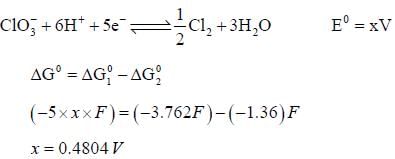
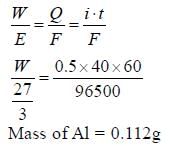
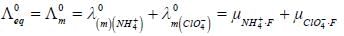
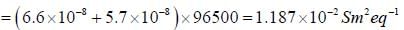
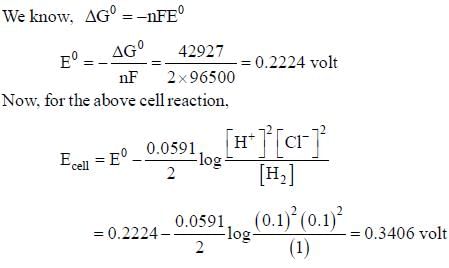
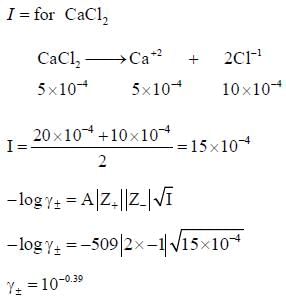
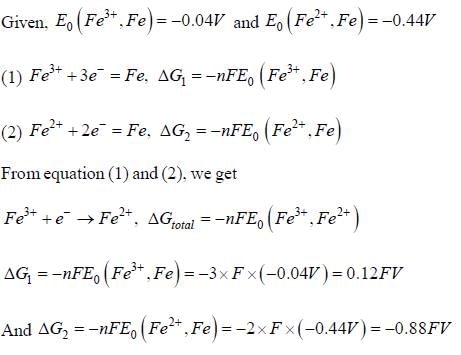
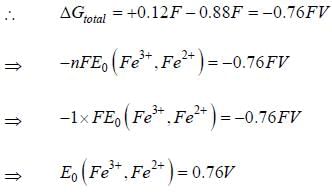
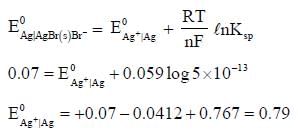
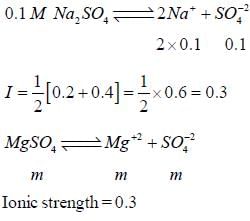
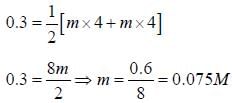
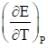 = 0 then |ΔH| > |nFE| endothermic reaction (Wrong relation)
= 0 then |ΔH| > |nFE| endothermic reaction (Wrong relation)