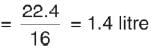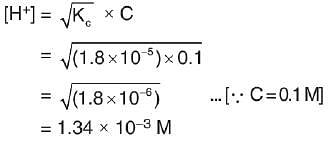EmSAT Chemistry Mock Test- 2 - EmSAT Achieve MCQ
30 Questions MCQ Test Mock Tests for EmSAT Achieve 2024 - EmSAT Chemistry Mock Test- 2
The nature of inter-molecular forces among benzene molecules is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The addition of HCN to a carbonyl compound is an example of
Sulphur trioxide is prepared by the following two reactions:-
S8(s) + 8 O2(g) —→ 8 SO2(g)
2SO2(g) + O2(g) —→ 2 SO3(g)
How many grams of SO3 are produced from 1 mole S8?
How many elements are more electropositive than Cl?
Be, F, O, S, P, Au, H, Na
How many elements have more ionisation energy as compared to their next higher atomic number element?
Na, Mg, Al, Si, P, S, Cl, Ar
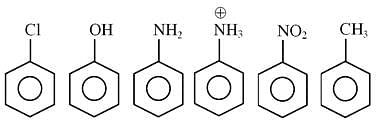
How many of the following compounds are more reactive than benzene towards electrophilic substitution.
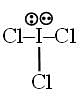
Number of d-orbitals used in the hybridisation of ICl3 is = x and number of lone pair at central atom = y find x + y = ?
When primary amine is heated with CS2 in presence of excess of mercuric chloride, it produce isothiocyanate. This reaction is known as
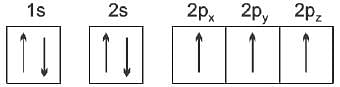
Total number of electrons in all the p-orbitals of bromine will be
Ionization constant of acetic acid is 1.8 * 10-5. The concentration of H+ ions in 0.1 M solution is
On heating one end of a piece of metal, tlhe other end becomes hot because of