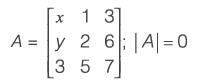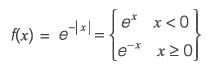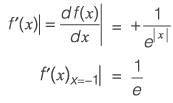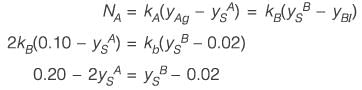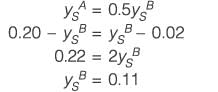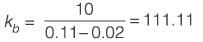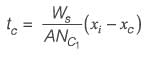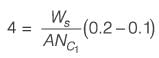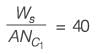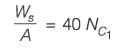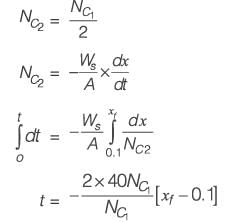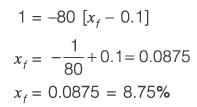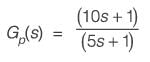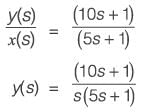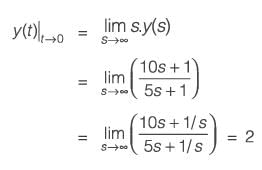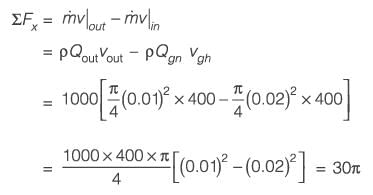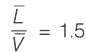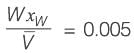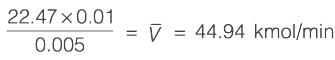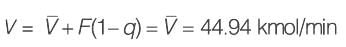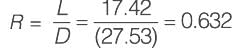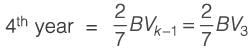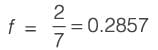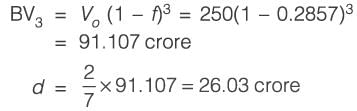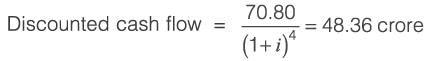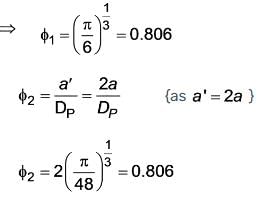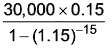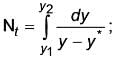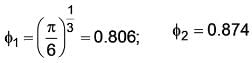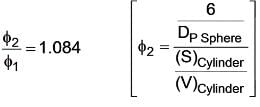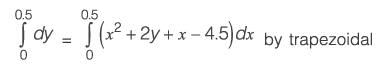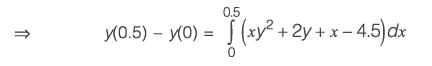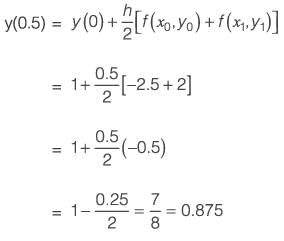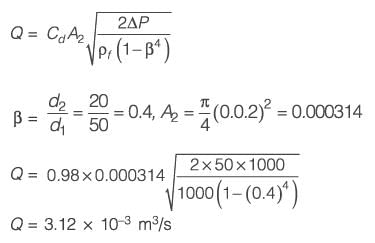GATE Chemical Engineering Mock Test - 1 - GATE Chemical Engineering MCQ
30 Questions MCQ Test GATE Chemical Engineering 2025 Mock Test Series - GATE Chemical Engineering Mock Test - 1
Identify the sentence with correct punctuation:
In a classroom of NIT Jaipur, the ratio of boys to girls is 3/4.
If there are 84 students in total, how many boys and how many girls are there?
If there are 84 students in total, how many boys and how many girls are there?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Choose the synonym that fits best in the blank:
"After the long hike, we felt completely ________ and needed to rest."
"After the long hike, we felt completely ________ and needed to rest."
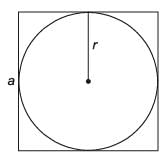
A sphere of radius r cm is packed in a box of cubical shape.
What should be the minimum volume (in cm3) of the box that can enclose the sphere?
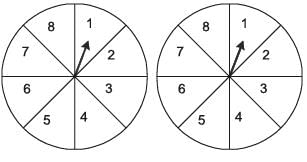
A game consists of spinning an arrow around a stationary disk as shown below. When the arrow comes to rest, there are eight equally likely outcomes. It could come to rest in any one of the sectors numbered 1, 2, 3, 4, 5, 6, 7 or 8 as shown. Two such disks are used in a game where their arrows are independently spun.
What is the probability that the sum of the numbers on the resulting sectors upon spinning the two disks is equal to 8 after the arrows come to rest?
Consider the following inequalities.
(i) 3p - q < 4
(ii) 3q - p < 12
Which one of the following expressions below satisfies the above two inequalities?
Given matrix  the ordered pair (x, y) for which det(A) = 0 is
the ordered pair (x, y) for which det(A) = 0 is
Let f(x) = e-|x|, where x is real. The value of df/dx at x = -1 is
For a single component system at vapor-liquid equilibrium, the extensive variables A, V, S and N denote the Helmholtz free energy, volume, entropy, and number of moles, respectively, in a given phase. If superscripts (v) and (/) denote the vapor and liquid phase, respectively, the relation that is NOT CORRECT is
The energy/heat required to completely vapourize 10 g of water beginning at 0º C. (The heat capacity of water is 4.2 J/g.K and the ΔH vapourization of water is 2260 kJ/kg).
Consider turbulent flow in a pipe under isothermal conditions. Let r denote the radial coordinate and z denote the axial flow direction. On moving away from the wall towards the center of the pipe, the rz-component of the Reynolds stress
Consider interphase mass transfer of a species S between two immiscible liquids A and B. The interfacial mass transfer coefficient of S in liquid A is twice of that in liquid B. The equilibrium distribution of S between the liquids is given by yAS = 0.5 yBS, where yAS and yBS are the mole-fractions of S in A and B, respectively. The bulk phase mole fraction of S in A and B is 0.10 and 0.02, respectively. If the steady-state flux of S is estimated to be 10 kmol h-1 m-2, the mass transfer coefficient of S in A is ______ kmol h-1 m-2 (rounded off to one decimal place).
A wet solid containing 20% (w/w) moisture (based on mass of bone-dry solid) is dried in a tray-dryer. The critical moisture content of the solid is 10% (w/w). The drying rate (kg m-2 s-1) is constant for the first 4 hours, and then decreases linearly to half the initial value in the next 1 hour. At the end of 5 hours of drying, the percentage moisture content of the solid is ________ % (w/w) (rounded off to one decimal place).
A process described by the transfer function
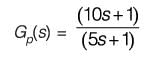
is forced by a unit step input at time t = 0. The output value immediately after the step input (at t = 0+) i s _________(rounded off to the nearest integer).
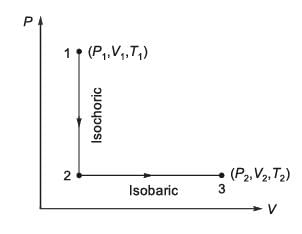
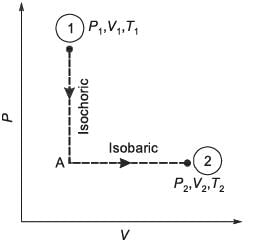
N moles of an ideal gas undergo a two-step process as shown in the figure. Let P, V, and T denote the pressure, volume, and temperature of the gas, respectively. The gas, initially at state-1 (P1, V1, T1), undergoes an isochoric (constant volume) process to reach state-A, and then undergoes an isobaric (constant pressure) expansion to reach state-2 (P2, V2, T2). For an ideal gas, CP - CV = NR, where CP and CV are the heat capacities at constant pressure and constant volume, respectively, and assumed to be temperature independent. The heat gained by the gas in the two-step process is given by
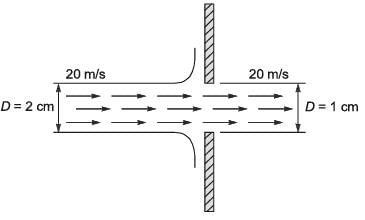
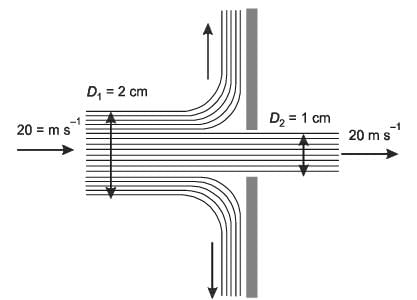
A horizontal cylindrical water jet of diameter D1 = 2 cm strikes a vertical solid plate with a hole of diameter D2 = 1 cm, as shown in the figure. A part of the jet passes through the hole and the rest is deflected along the plate. The density of water is 1000 kg m-3. If the speed of the jet is 20 m s-1, the magnitude of the horizontal force, in N, required to hold the plate stationary is
Which of the following statements is correct regarding the heat transfer mechanisms of convection and radiation?
In a chemical reaction following Michaelis-Menten kinetics, the reaction rate at high substrate concentrations approaches:
An equimolar binary mixture is to be separated in a simple tray-distillation column. The feed rate is 50 kmol min-1. The mole fractions of the more volatile component in the top and bottom products are 0.90 and 0.01, respectively. The feed as well as the reflux stream are saturated liquids. On application of the McCabe-Thiele method, the operating line for the stripping section is obtained as
y = 1.5 x - 0.005
where y and x are the mole fractions of the more volatile component in the vapor and liquid phases, respectively. The reflux ratio i s ______ (rounded off to two decimal places).
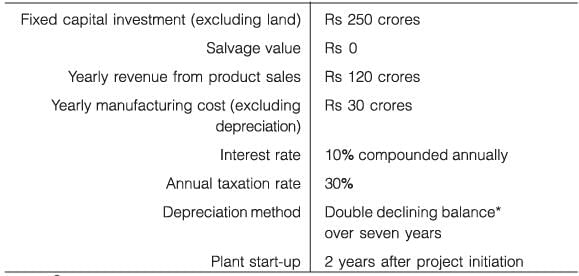
Information for a proposed greenfield project is provided in the table. The discounted cash flow for the fourth year is Rs_______crores (rounded off to one decimal place).
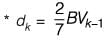 k: years post start-up d: Depreciation amount BV: Book value
k: years post start-up d: Depreciation amount BV: Book value
In a jaw crusher, material of size 50 mm is crushed to a product of 5 mm and energy required comes at ‘E1’ kW.hr/tons while when a feed of size 25 mm is crushed to a product of size 2.5 mm, Energy required is ‘E2’ in kW.hr/tons. If Kick’s law is used what is the ratio of E1/E2. (Upto one decimal place).
For a cube with side ‘a’ the sphericity comes out to be 'ϕ1' if the side of cube is changed to ‘2a’ and sphericity becomes 'ϕ2'. what will be the ratio of
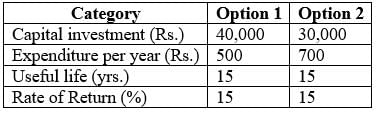
What is the total annual cost for option 2.
(in rupees to the nearest integer) ………………
Solute B has to be extracted from a solution containing solute B and solvent A with 30% of B by weight using solvent C. 1.5 kg of solution is feed with 1 kg of solvent C. Distribution coefficient is given as:
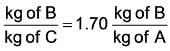
Solvent A and B are immiscible.
Calculate the amount of solute B in extract phase (in kg, upto two decimal place)
A solution is used to reduce the concentration of gas A from 13% to 0.4% by absorption. Calculate the number of overall transfer units on the gas A phase basis.
Assume : Absorption is accompanied with irreversible chemical reaction ....................
A cube of 1 unit length has a sphericity 'ϕ1' and a cylinder with height equals 10 diameter of 1 unit length has sphericity 'ϕ2' Calculate ϕ2/ϕ1. (Upto two decimal places).
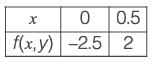
The equation dy/dx = xy2 + 2y + x - 4.5 with the initial condition y{x = 0) = 1 is to be solved dx using a predictor-corrector approach. Use a predictor based on the implicit Euler’s method and a corrector based on the trapezoidal rule of integration, each with a full-step size of 0.5. Considering only positive values of y, the value of y at x = 0.5 is __________ (rounded off to three decimal places).
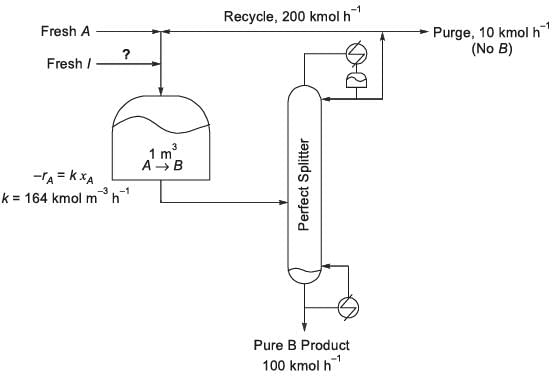
Consider the process flowsheet in the figure. An irreversible liquid-phase reaction A → B (reaction rate -rA = 164 xA kmol m-3 h-1) occurs in a 1 m3 continuous stirred tank reactor (CSTR), where xA is the mole fraction of A. A small amount of inert, I, is added to the reactor. The reactor effluent is separated in a perfect splitter to recover pure B product down the bottoms and a B-free distillate. A fraction of the distillate is purged and the rest is recycled back to the reactor. At a particular steady state, the product rate is 100 kmol h-1, the recycle rate is 200 kmol h-1 and the purge rate is 10 kmol h-1. Given the above information, the inert feed rate into the process is ______ kmol h-1 (rounded off to two decimal places).
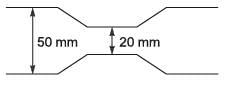
A venturi meter (venturi coefficient, Cv = 0.98) is connected to a pipe of inner diameter 50 mm. Water (density 1000 kg m-3) is flowing through the pipe. The pressure-drop measured across the venturi meter is 50 kPa. If the venturi throat diameter is 20 mm, the estimated flow rate of water is ______ x 10-3 m3 s-1 (rounded off to two decimal places).