Inorganic Chemistry MCQ - Biotechnology Engineering (BT) MCQ
21 Questions MCQ Test Mock Test Series of IIT JAM Biotechnology 2025 - Inorganic Chemistry MCQ
In which of the following pairs of molecules / ions both the species are not likely to exist?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following complexes (0.1 mol), during precipitation with Ag+ ion, will result the minimum prepitate of AgCl?
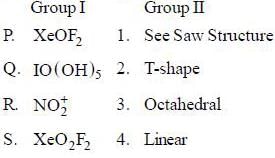
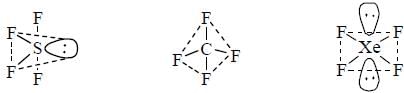
The collect set of match between molecules of group I and their shapes in Group II is
Which of the following compounds are diamagnetic in nature ?
Which of the following compounds will not contains spin-only magnetic moment 2.82 B.M is are
In the structure of 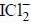 the number of lone pair present on centeral atom is _____.
the number of lone pair present on centeral atom is _____.
The change in oxidation state of Cobalt in the following reaction is ______.
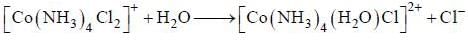
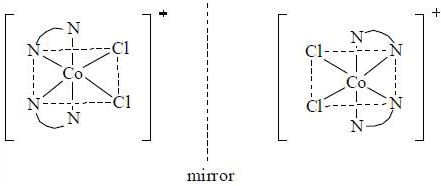
The number of optically active Isomers possible for [Co(en)2 Cl2]+ complex is ____ .
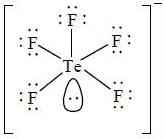
Based on VSEPR theory, the number of 90° Cl–Te–Cl angles in 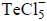 is ______ .
is ______ .
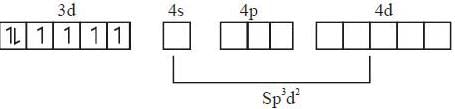
The number of unpaired elections in the coordination complex anion [CoF6]3- is _____.
The number of species that undergoes disproportionation in an alkaline medium is ______.
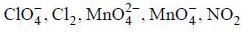


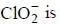

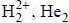 comes out to be zero.
comes out to be zero.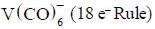
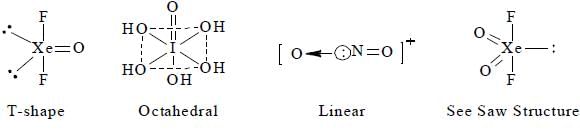
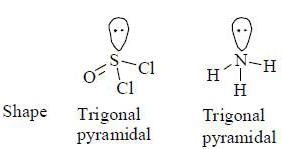
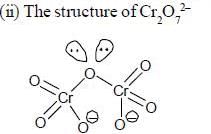
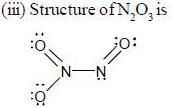

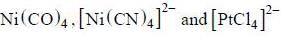 are diamagnetic in nature their spin only magnetic moment equation zero.
are diamagnetic in nature their spin only magnetic moment equation zero.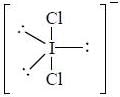
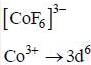
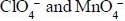 are in their maximum oxidation state does not undergoes disproportionatioin reaction while
are in their maximum oxidation state does not undergoes disproportionatioin reaction while 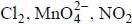 species undergoes disproportionation in alkaline medium.
species undergoes disproportionation in alkaline medium.









