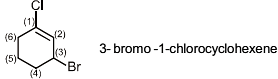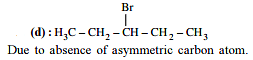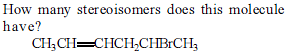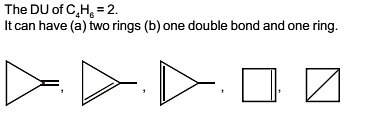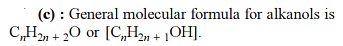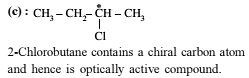Isomerism - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Isomerism - 1
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the specific rotation if its observed rotation is given as 3x, its length is given as x and density is given as 3/y?
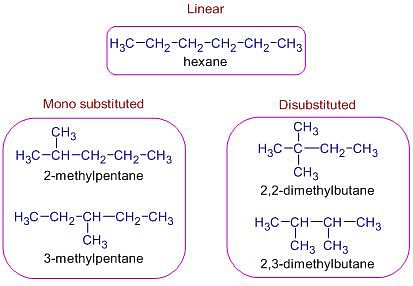
How many number of all structurally isomeric dienes with molecular formula C5H8 are possible :
S1 : Trans-But-2-ene has higher boiling point than cis-But-2-ene.
S2 : 1, 4-Dichlorobenzene has zero dipole moment.
S3 : Trans cyclodecene is more stable as compare to cis-cyclodecene.
S4 : Trans 1, 2-Dibromoethene is more soluble in water than cis-1, 2-Dibromoethene
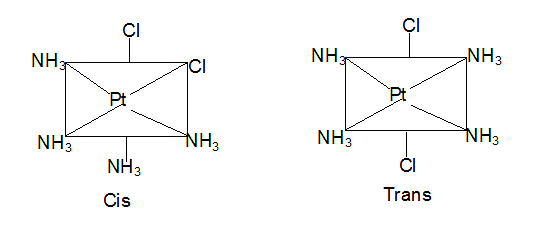
Which statement(s) is/are true about the relation between the following compounds?

(I) a and b are tautomers
(II) b and c are resonating structures
(III) a and c are resonating structures
(IV) a and c are tautomers
Which of the following compound has zero dipole moment in one of the stable conformations
The number of structurally isomeric esters with molecular formula C5H10O2 are.
The total number of cyclic isomers possible for a hydrocarbon with the molecular formula C4H6 is / are :
What is the degree of unsaturation in a compound with molecular formula C9H6N4?
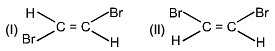
Which of the following physical property is greater for (I) compound.
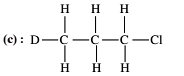
Out of the following, the alkene that exhibits optical isomerism is.
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|