Jharkhand TGT (JSSC) Mock Test - 4 - Jharkhand (JSSC) PRT/TGT MCQ
30 Questions MCQ Test Jharkhand (JSSC) TGT Exam Mock Test Series 2025 - Jharkhand TGT (JSSC) Mock Test - 4
Find the equation of the hyperbola whose vertices are 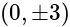 and the eccentricity is
and the eccentricity is 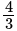 ?
?
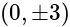 and the eccentricity is
and the eccentricity is 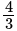 ?
?If the plane 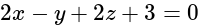 has the distances
has the distances 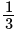 and
and 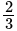 units from the planes
units from the planes 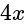
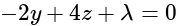 and
and 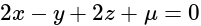 , respectively, then the maximum value of
, respectively, then the maximum value of 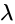
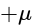 is equal to:
is equal to:
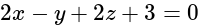 has the distances
has the distances 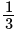 and
and 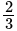 units from the planes
units from the planes 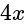
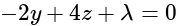 and
and 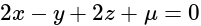 , respectively, then the maximum value of
, respectively, then the maximum value of 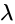
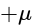 is equal to:
is equal to:The equation of the normal at the point 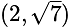 to the hyperbola
to the hyperbola 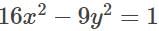 is:
is:
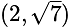 to the hyperbola
to the hyperbola 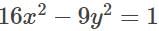 is:
is:If A and B are two sets then A ∩ (B ∪ A)c is equal to-
The graph of a linear equation in two variables is:
Find the area of the region bounded by y2=4x, x=1, x=4, and x-axis in the first quadrant.
A window in a building is at a height of 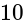 meters from the ground. The angle of depression of a point
meters from the ground. The angle of depression of a point 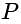 on the ground from the window is
on the ground from the window is  . The angle of elevation of the top of the building from the point
. The angle of elevation of the top of the building from the point 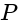 is
is 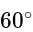 . What is the height of the building?
. What is the height of the building?
According to Dalton's law of partial pressures, (where pb = Barometric pressure, pa = Partial pressure of dry air, and pv = Partial pressure of water vapour).
The number of images of an object placed between two parallel mirrors is:
A full length image of a distant tall building can definitely be seen by using:
In Huygens principle, the relation between wavefront and the ray of light is:
The Peak Inverse Voltage rating of a crystal diode is ________________ that of equivalent vacuum diode.
A wire of length 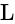 is drawn such that its diameter is reduced to half of its original diameter. If the initial resistance of the wire were
is drawn such that its diameter is reduced to half of its original diameter. If the initial resistance of the wire were 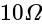 , its new resistance would be:
, its new resistance would be:
The heat given to an ideal gas in isothermal conditions is used to:
The ion with the strongest polarizing capacity is:
The volume of hexagonal ice lattice is given by:
Which of the following is a thermosetting polymer?
When beryllium is bombarded with α -particles, extremely penetrating radiations that cannot be deflected by an electrical or magnetic field are given out. These are
In polysaccharides, monosaccharaides are joined by:
The compound formed when ethyl bromide is heated with dry silver oxide is:
Which of the following is not a condensation polymer?
Which one among the following most correctly determines the atomic number of an element?
PA = (235 y -125 xy)mm of Hg where PA is partial pressure of A
x is mole fraction of B in liquid phase in the mixture of two liquids A and B and y is mole fraction of A in vapour phase, 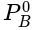 then in mm of Hg is:
then in mm of Hg is:


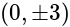 with
with 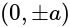 we get
we get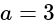
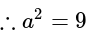
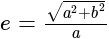
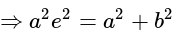
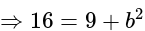
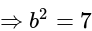
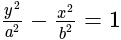
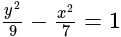 .
.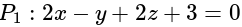
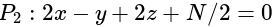
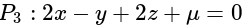
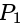 and
and 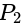 is
is 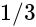 .
.
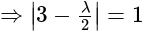
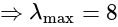
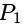 and
and 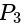 is
is 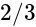 .
.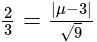
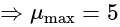
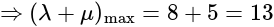
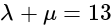 .
.
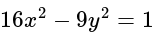 .
. '.
'.
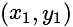 and having slope
and having slope 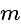 is:
is: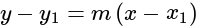
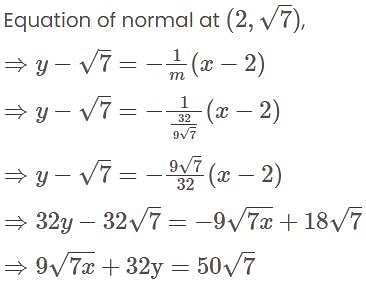
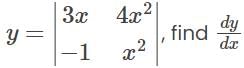
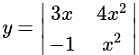
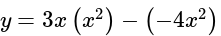
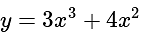
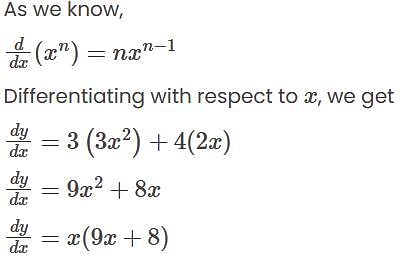

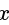 , we get
, we get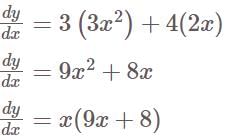
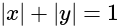 is:
is: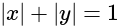 is as follows.
is as follows.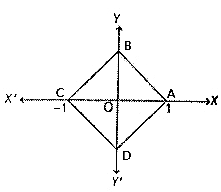
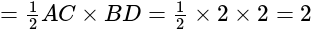 sq.units
sq.units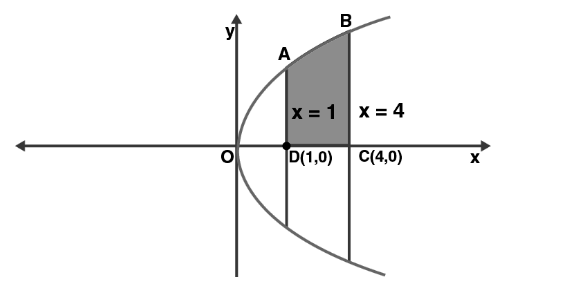
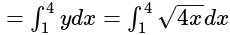
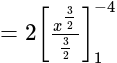
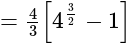
 sq. units
sq. units then the value of
then the value of  is:
is: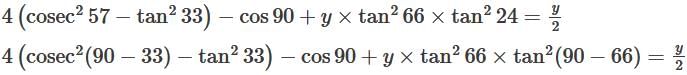

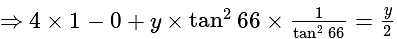
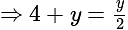
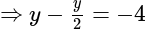
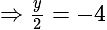

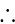 The value of
The value of 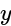 is
is 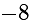 .
.
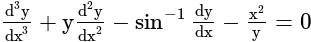
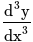 .
.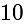 meters
meters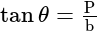
 perpendicular or height
perpendicular or height base
base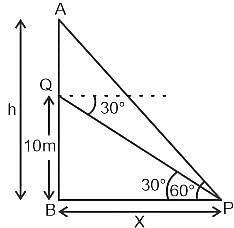
 .
.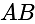
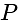 at a distance of
at a distance of 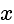 meter.
meter.
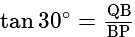
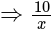

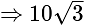
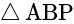

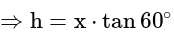
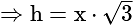


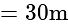
 , keeping volume constant,
, keeping volume constant,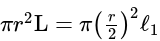

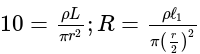
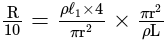
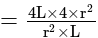
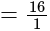
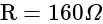
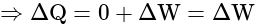
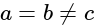 and
and 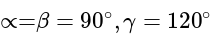
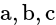 are the axial distances and
are the axial distances and 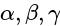 are the axial angles.
are the axial angles.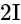 and altitude
and altitude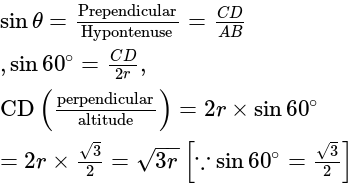
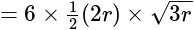
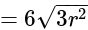
 Base area
Base area  Height
Height 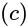 .
.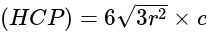 ,
, 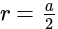
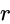 in equation (iii), we get
in equation (iii), we get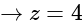
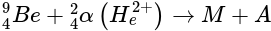 (Atom release)
(Atom release)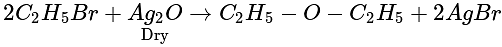
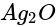 then alcohol is formed
then alcohol is formed 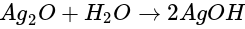
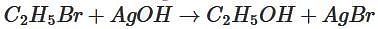
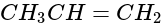 produces:
produces: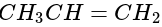
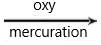
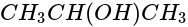
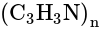 . Although it is thermoplastic it does not melt under normal conditions, since it degrades before melting at above
. Although it is thermoplastic it does not melt under normal conditions, since it degrades before melting at above 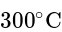 . Almost all Polyacrylonitrile resins are copolymers made from mixtures of monomers, with acrylonitrile as the main component. As it is a non condensation polymer.
. Almost all Polyacrylonitrile resins are copolymers made from mixtures of monomers, with acrylonitrile as the main component. As it is a non condensation polymer.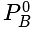 in mm of Hg
in mm of Hg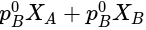 ......(v)
......(v)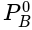 = Vapour pressure of pure A
= Vapour pressure of pure A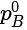 =Vapour pressure of pure B
=Vapour pressure of pure B














