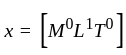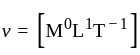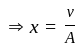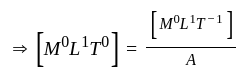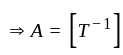MP Police SI (Technical) Mock Test - 9 - MP Police SI MCQ
30 Questions MCQ Test MP Police SI Mock Test Series 2025 - MP Police SI (Technical) Mock Test - 9
A dealer gains 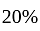 by selling an article at
by selling an article at 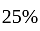 discount on its marked price. If the cost price of the article is decreased by
discount on its marked price. If the cost price of the article is decreased by 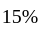 , how much discount percentage should he now give on the same marked price so as to earn the same percentage of profit as before?
, how much discount percentage should he now give on the same marked price so as to earn the same percentage of profit as before?
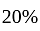 by selling an article at
by selling an article at 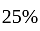 discount on its marked price. If the cost price of the article is decreased by
discount on its marked price. If the cost price of the article is decreased by 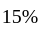 , how much discount percentage should he now give on the same marked price so as to earn the same percentage of profit as before?
, how much discount percentage should he now give on the same marked price so as to earn the same percentage of profit as before?Nitrogen has no d-orbital in its valence shell and therefore it cannot
1) Exhibits the oxidation state of +5
2) Have covalency greater than 3
3) Exhibit orbital Hybridization
4) Form oxides with oxidation states greater than +5
Select the correct answer using codes given below:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A manufacturer makes 600 articles at a cost of Rs. 5 per article. He fixes the selling price such that if only 500 articles are sold, he would make a profit of 30% on his outlay. However 60 articles get spoilt and he was able to sell 540 articles at this price. Find his actual profit percent.
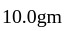
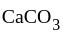 on heating gave
on heating gave 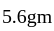 of
of 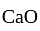 and
and 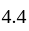 gm of
gm of 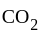 , given data support the law of:
, given data support the law of:
The marked price of a TV is Rs. 25,000. The shopkeeper gives a successive discount of 15% and r% to the customer. If the customer pays Rs. 17,000 for the TV, then the value of r is:
The reaction 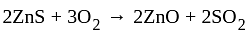 in the metallurgical process of zinc is called:
in the metallurgical process of zinc is called:
The most abundant metal in the earth’s crust is:
The percentage error in the measurement of mass and speed are 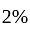 and
and 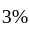 respectively. The error in the estimate of kinetic energy obtained by measuring mass and need will be:
respectively. The error in the estimate of kinetic energy obtained by measuring mass and need will be:
Which of the following is yielded when Ethylene glycol is treated with phosphorus tri-iodide?
Let 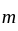 and
and 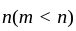 be the roots of the equation
be the roots of the equation 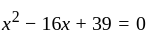 . If four terms
. If four terms 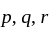 and
and 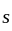 are inserted between
are inserted between 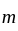 and
and 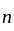 to form an
to form an 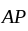 , then what is the value of
, then what is the value of 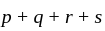 ?
?
A number is first decreased by  and then increased by
and then increased by  . If the number so obtained is
. If the number so obtained is 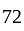 less than the original number, then what is the value of the original number?
less than the original number, then what is the value of the original number?
Akash and Babul started a business by investing their amount in the ratio of 2 : 3. If at the end of the babul got Rs. 2400 as a profit, what is the profit amount get by Akash at the end of the year?
P and Q invested in a business. The profit earned was divided in the ratio 2 : 3. If P invested Rs 40000, the amount invested by Q is:
Which of the following gases is produced during calcination?
Notice the equation 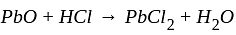
The balance of the above equation requires_____________ moles of 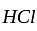 .
.
What lenses are used by people having shortsightedness?
Three equal resistors connected in series across a potential difference and together dissipate 2000 J heat in time t. If the same resistors are connected parallel across the same potential difference, then the heat dissipated in the same time will be:
During electrolytic refining of nickel, _______ is taken as anode.
Direction: In the following question two equation numbered I and II are given. Solve the equation and answer the question:
I. 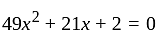
II. 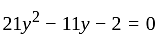
Which of the following terms does not represent electric power in a circuit?
Which one of the following statements is true for the speed 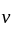 and the acceleration
and the acceleration 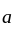 of a particle executing simple harmonic motion?
of a particle executing simple harmonic motion?
If a set A contains 3 elements and another set B contains 6 elements, then what is the minimum number of elements that (A∪B) can have?
What is the colour of the coating formed on copper when it reacts with moist carbon dioxide?
What is the color of the flame produced by burning natural gas?
Which of the following will not react with hrdroxyl ions by nucleophilic attack?
What is the function of an important part of the human eye, the iris?
Which of the following metal and alloy pairs is not a correct combination?
i. Steel and Iron
ii. Bronze and Tin
iii. Brass and Copper
The position of a particle at any time 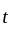 is given by the relation
is given by the relation 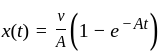 where
where 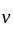 is the velocity. Then what will be the dimension of
is the velocity. Then what will be the dimension of 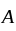 ?
?


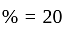
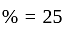
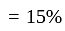
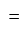 Marked price
Marked price 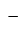 Discount
Discount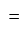 Cost price
Cost price 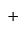 profit
profit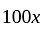 .
.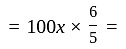 Rs.
Rs. 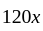
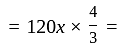 Rs.
Rs. 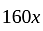
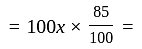 Rs.
Rs. 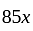
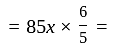 Rs.
Rs. 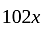
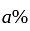 .
.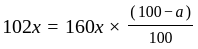
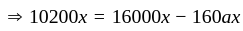
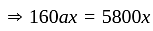
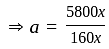
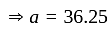
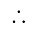 He should give
He should give 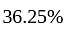 discount on decreased cost price.
discount on decreased cost price.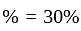
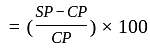
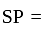 selling price
selling price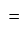 Rs.
Rs. 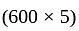
 Rs. 3000
Rs. 3000 Rs.
Rs. 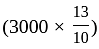
 Rs. 3900
Rs. 3900 Rs.
Rs. 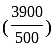
 Rs.
Rs. 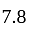
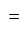 Rs.
Rs. 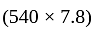
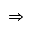 Rs. 4212
Rs. 4212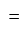 Rs.
Rs. 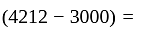 Rs. 1212
Rs. 1212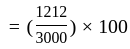
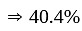
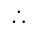 Profit percent is
Profit percent is 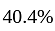
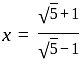 and
and 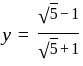 , then find the value of
, then find the value of 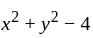 .
.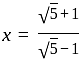 and
and 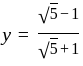
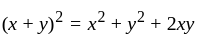
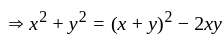
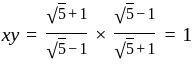
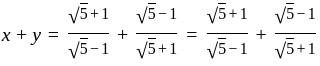
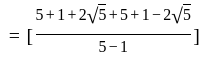
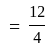
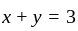
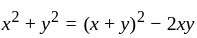 , we get
, we get 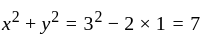
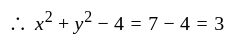
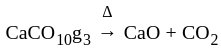

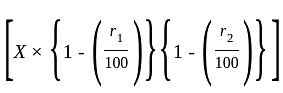
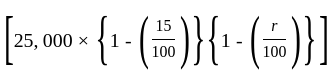
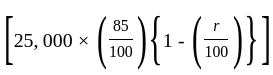
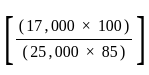
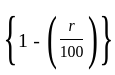
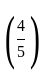
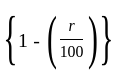
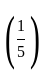
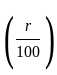
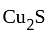 (chalcocite) and
(chalcocite) and 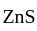 (sphalerite), balanced equations for the roasting are:
(sphalerite), balanced equations for the roasting are: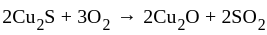
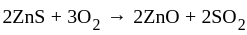
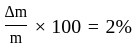
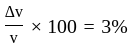
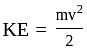
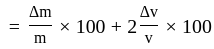
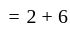
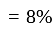
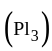 is an unstable red solid which reacts violently with water. It is a common misconception that
is an unstable red solid which reacts violently with water. It is a common misconception that  is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides.
is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides.
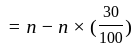
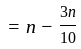
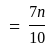
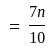

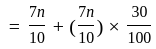
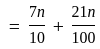
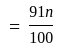
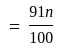
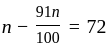
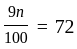
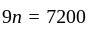
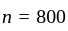
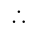 The value of original number is
The value of original number is 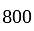 .
.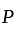 and
and 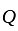 invested in a business. The profit earned was divided in the ratio
invested in a business. The profit earned was divided in the ratio 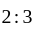 .
.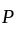 to
to 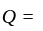 ratio of their profit
ratio of their profit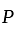 invested Rs. 40000 and amount invested by
invested Rs. 40000 and amount invested by 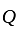 is
is 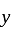 .
.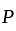 to
to 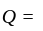 ratio of their profit
ratio of their profit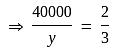
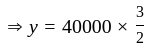
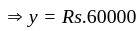
 The amount invested by
The amount invested by 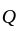 is Rs.
is Rs. 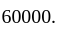
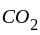 is produced during calcination. It involves heating when the volatile matter escapes leaving behind the metal oxide.
is produced during calcination. It involves heating when the volatile matter escapes leaving behind the metal oxide.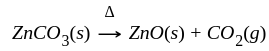
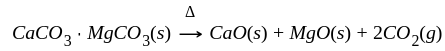
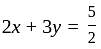 and
and 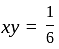 , then what is the value of
, then what is the value of 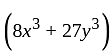 ?
?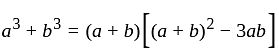
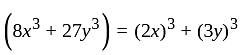
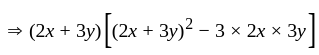
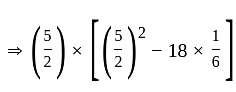
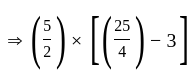
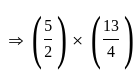
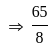
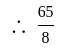 is the required answer.
is the required answer.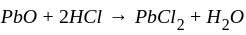 is the balanced equation.
is the balanced equation.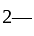 moles of
moles of 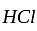 .
. watt
watt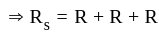
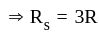 ....(1)
....(1)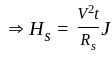
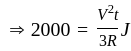
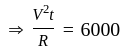 ...........(2)
...........(2)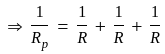
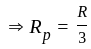 .................(3)
.................(3)
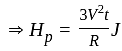 .................(4)
.................(4)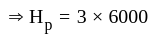
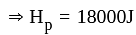
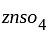 used as electrolyte.
used as electrolyte.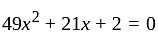
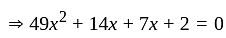
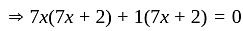
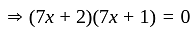
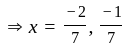
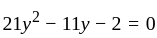
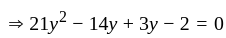
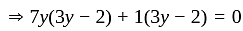
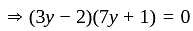
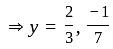
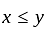
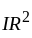 does not represent electric power in a circuit.
does not represent electric power in a circuit.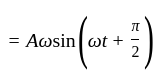
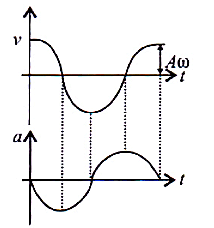
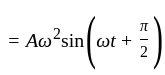
 is maximum,
is maximum,  is zero.
is zero.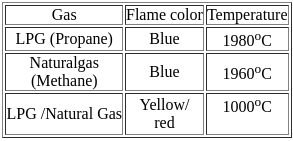
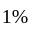 carbon and may contain some other elements like manganese.
carbon and may contain some other elements like manganese.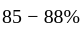 copper,
copper, 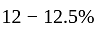 tin, and with the addition of some other metals like aluminum, manganese, zinc or nickel in small proportions.
tin, and with the addition of some other metals like aluminum, manganese, zinc or nickel in small proportions.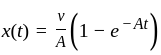
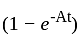 is a constant value and will have no dimension.
is a constant value and will have no dimension.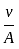 will be equal to the dimension of
will be equal to the dimension of 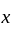 .
.