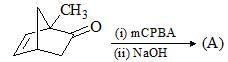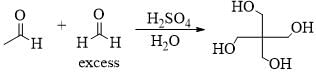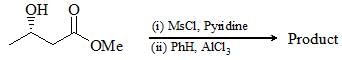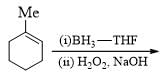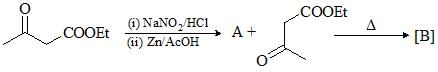Major Organic Chemistry - Chemistry MCQ
30 Questions MCQ Test Mock Test Series for IIT JAM Chemistry - Major Organic Chemistry
An organic compound (MF = C8H10O) exhibit the following 1H NMR spectral data:
δ 2.5 (3H, s), 3.8 (3H, s), 6.8 (2H, d, J = 8 Hz), 7.2 (2H, d, J = 8Hz), The compound is:
δ 2.5 (3H, s), 3.8 (3H, s), 6.8 (2H, d, J = 8 Hz), 7.2 (2H, d, J = 8Hz), The compound is:
Which chemical test will distinguish the compounds shown below?
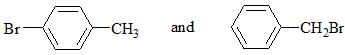
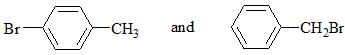
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
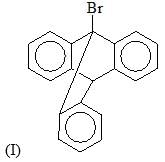
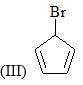
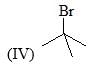
The reactivity of compound I-IV with maleic anhydride (V) follows the order:
(I)  (II)
(II) 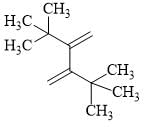 (III)
(III) 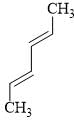 (IV)
(IV) 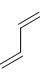 (V)
(V) 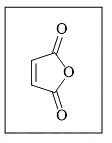
(I)
 (II)
(II)  (III)
(III)  (IV)
(IV)  (V)
(V) 
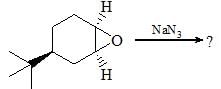
Consider the following:
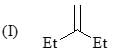
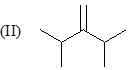
Order of stretching frequency in IR-spectroscopy would be:
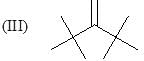
The conditions A-B, required for the following pericyclic are:
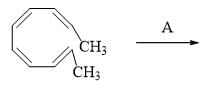


In the following three step transformation, identify the correct combination product P, Q and R:


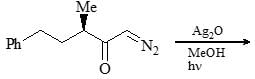
Consider the given chemical reaction and predict B and C:
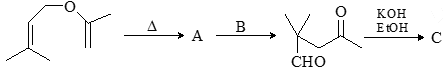
Consider the following chemical reaction and choose the correct product:
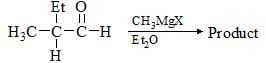
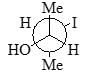
Which of the following is diastereomer of the given compound?
Consider the following reactions for a compound with molecular formula C10H16:

C10H16 is a terpene called Myrcene and follows is isoprene rule. Hence, correct structure of Myrcene among three possible isomers of C10H16 is:
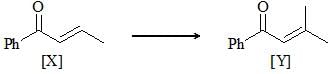
For the conversion of X to Y, the appropriate sequence of reaction is:
Consider the following chemical reaction & predict the major product. [Major product has two geometrical isomers which are inter-convertible in presence of light]:

The correct order of the basicity of following compounds is:
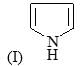
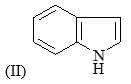
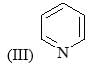

Correct order of rate of hydrolysis for following compounds is:

Predict the product for the following sugar functionalization reaction:
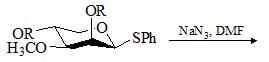

Which of the following bases would give the high percentage of alk-1-ene from 2-bromo-2,3-dimethylbutane?
Choose the major product formed in given chemical reaction sequence:
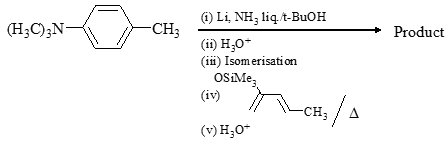
What are the most likely products of the reaction shown below?
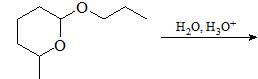
Major product formed in the following chemical reaction would be?
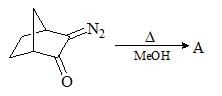
Consider the following chemical reaction and predict the product ‘D’:

Consider the following chemical reaction sequence. Predict the product ‘Z’:
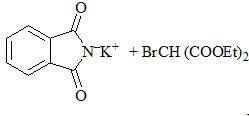
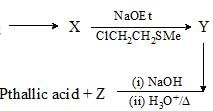
|
2 docs|25 tests
|