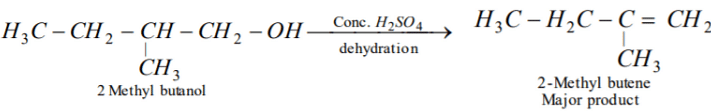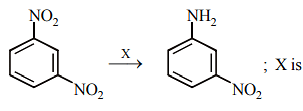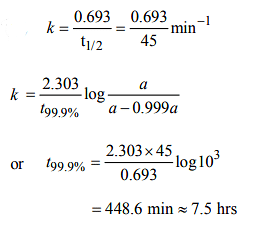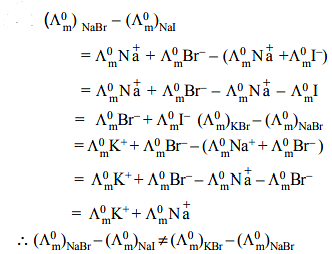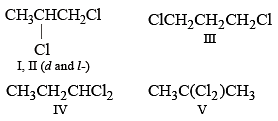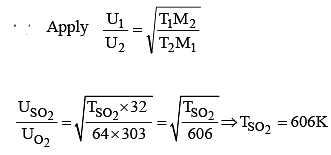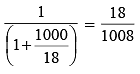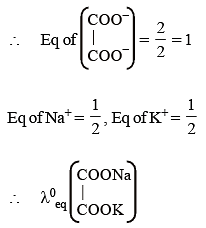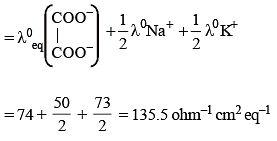NEET Practice Test - 14 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Practice Test - 14
The dehydration of 2-methyl butanol with conc. H2SO4 gives :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
For how many orbitals, the quantum numbers n = 3, l = 2, m = +2 are possible?
Among the following organic acids, the acid present in rancid butter is:
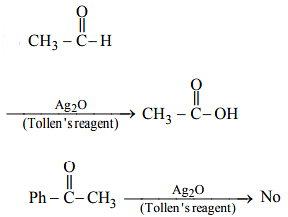
Which of the following reagents is used for the separation of acetaldehyde from acetophenone?
A first order reaction is half completed in 45 minutes. How long does it need for 99.9% of the reaction to be completed?
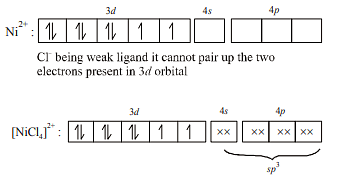
The complex showing a spin-only magnetic moment of 2.82 B.M. is :
Among the lanthanides, the one obtained by synthetic method is
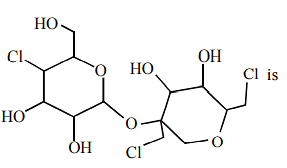
A compound is formed by substitution of two chlorine for two hydrogens in propane. The number of possible isomeric compounds is
At what temperature does the rms velocity of SO2 be the same as that of O2 at 303K ?
Which is not obtained when metal carbides react with H2O?
Each of the following solids show, the Frenkel defect except
The mole fraction of the solute in one molal aqueous solution is:
A process has ΔH = 200 J mol–1 and DS = 40 JK–1 mol–(1) Out of the values given below, choose the minimum temperature above which the process will be spontaneous:
In sodium fusion test of organic compounds, the nitrogen of the organic compound is converted into
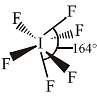
The pair of species having identical shapes for molecules of both species is
Which complex of Co2+ will have the weakest crystal field splitting –
What is the percentage of lanthanide metal in mischmetal?
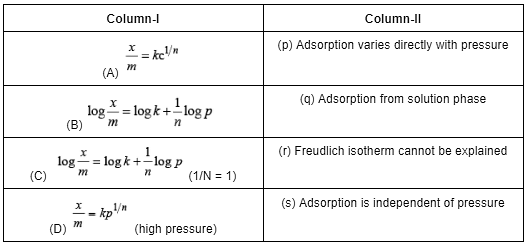
Given the ionic conductance of  ,and Na+ are 74, 50 and 73 cm2 ohm-1 eq–1, respectively. The equivalent conductance at infinite dilution of the salt
,and Na+ are 74, 50 and 73 cm2 ohm-1 eq–1, respectively. The equivalent conductance at infinite dilution of the salt 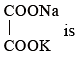
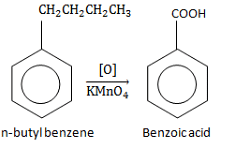
Which of the following will have the fastest rate of reaction with Br2/FeBr3?
An ideal gas obeying kinetic theory of gasses can be liquefied if:
|
1 videos|26 docs|111 tests
|
|
1 videos|26 docs|111 tests
|