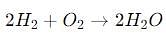OAVS TGT Science Mock Test - 10 - OTET MCQ
30 Questions MCQ Test OAVS TGT Mock Test Series 2025 - OAVS TGT Science Mock Test - 10
If 9g of water is decomposed, it is always obtained –
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Identify the type of reaction: HCl + NaOH → NaCl + H2O
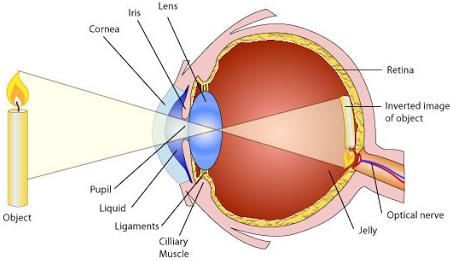
The light coming from an object enters the eye through:
Which of the following represents the correct sequence of stages in aerobic respiration?
Find the incorrect statement regarding cell organelles from the following:
Which of the following is not a digestive enzyme contained in the pancreatic juice?
i. Lipase
ii. Hydrochloric acid
iii. Mucus
iv. Trypsin
Excessive exposure of humans to UV-rays results in
(i) damage to immune system
(ii) damage to lungs
(iii) skin cancer
(iv) peptic ulcer
Which of the following plant has unisexual flowers?
Molecular weight of CuS04. 5H20 is equal to
[Cu = 63.5 u, S = 32 u, O — 16 u, H = 1 u]
What factor has led to increased waste generation according?
In the context of redox reactions, what happens to the substance that is reduced?
Which is not represented by 1 mole of nitrogen gas?
The phenomena of light responsible for the working of the human eye is
An emulsion is a colloidal solution formed by mixing
Two pea plants one with round green seeds (RRyy) and another with wrinkled yellow (rrYY) seeds produce FI progeny that have round yellow (RrYy) seeds. When F1 plants are selfed, the F2 progeny will have new combination of characters.
Choose the new combination from the following
(i) Round, yellow
(ii) Round, green
(iii) Wrinkled, yellow
(iv) Wrinkled, green
Two balls A and B, of masses m and 2m are in motion with velocities 2v and v respectively. Compare the force needed to stop them in the same time
The following chemical reaction shows the addition of chlorine to methane in the presence of sunlight:
CH4 + Cl2 → X
What is likely to be the product of the reaction represented by “X”?