Periodic properties - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Periodic properties
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The electronic configuration which is associated with highest first ionisation enthalpy is:
 atoms of
atoms of 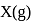 are converted into X+(g) by energy
are converted into X+(g) by energy  atoms of
atoms of  are converted into
are converted into  by energy
by energy  . , then ionization potential and electron affinity of
. , then ionization potential and electron affinity of  respectively are
respectively are
Match Column-I with Column-II and select the correct answer by the given codes.

The set of three elements having successive atomic numbers and having the ionization energies of 2372, 520 and  per mol is
per mol is
In a given energy level, the order of penetration effect of different orbitals is
What is the order of ionisation energies of the coinage metal
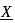 . What is
. What is 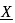 ?
?
The electronic contigurations of four elements are given below. Arrange these elements in the correct order of magnitude of their electron affinity
(i) 
(ii) 
(iii) 
(iv) 
Select the correct answer using the codes
Higher second ionisation enthalpies than expected are observed for
In second period of the long form of the periodic table, an element X has second lowest first ionization enthalpy and element Y has second highest first ionization enthalpy values. What are X and Y ?
Which among the following elements of 3nd period shows maximum tendency of forming dπ − pπ bond ?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


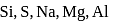 is
is period elements is
period elements is
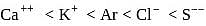
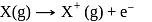
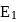 for
for  atoms
atoms atoms of
atoms of  have been ionized, by energy
have been ionized, by energy 
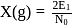 per atom
per atom
 for
for 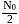 atoms
atoms is
is  per atom.
per atom.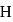 and He only out of which He is inert, hence, H behaves as a highly electropositive as well as electronegative.
and He only out of which He is inert, hence, H behaves as a highly electropositive as well as electronegative.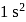 → Highest ionisation energy due to noble gas in nature.
→ Highest ionisation energy due to noble gas in nature.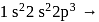 High electronegativity in nature due to small size and -1 oxidation state.
High electronegativity in nature due to small size and -1 oxidation state.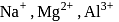 and
and 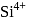 are isoelectronic. The order of their ionic size is
are isoelectronic. The order of their ionic size is
 -electron has to be removed. In the case of
-electron has to be removed. In the case of  , a
, a  electron is to be removed which is closer to the nucleus than the
electron is to be removed which is closer to the nucleus than the 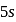 electron of
electron of  .
. of
of  of Ag.
of Ag. of
of  orbitals, the nuclear charge increases and therefore 5s
orbitals, the nuclear charge increases and therefore 5s  of
of  is more tightly held. Thus, the order of IE1 is Cu > Ag < Au.
is more tightly held. Thus, the order of IE1 is Cu > Ag < Au.
 has completely filled
has completely filled  -orbital, so highest energy is absorbed when it converts to
-orbital, so highest energy is absorbed when it converts to 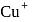 ion.
ion. -subshell. Since, the
-subshell. Since, the  -subshell after poor shielding, the atomic size will decrease as the nuclear charge increases.
-subshell after poor shielding, the atomic size will decrease as the nuclear charge increases.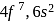 with half-filled outermost shell so nuclear pull is less and it does not show lanthanide contraction.
with half-filled outermost shell so nuclear pull is less and it does not show lanthanide contraction. has stable electronic configuration (1s2,2s22p6). If remove extra electrone that is present in inner shell, so need more energy to remove it.(insufficient amount of energy)
has stable electronic configuration (1s2,2s22p6). If remove extra electrone that is present in inner shell, so need more energy to remove it.(insufficient amount of energy) is
is  and that of
and that of  is
is  . Their second ionisation enthalpies are higher than expected because after removal of one electron chromium gets a half-filled electronic configuration and
. Their second ionisation enthalpies are higher than expected because after removal of one electron chromium gets a half-filled electronic configuration and  get a fully-filled
get a fully-filled 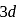 electronic configuration. These configuration are more stable than the partial-filled electronic configuration. So, it is very difficult to remove the second electron in both cases.
electronic configuration. These configuration are more stable than the partial-filled electronic configuration. So, it is very difficult to remove the second electron in both cases.














