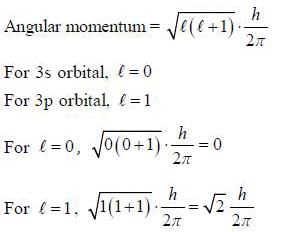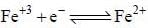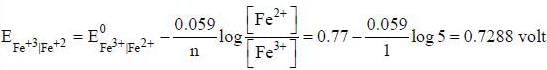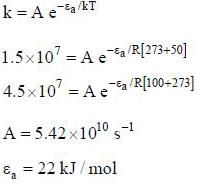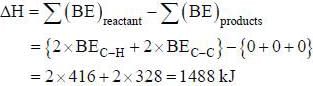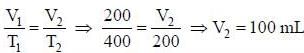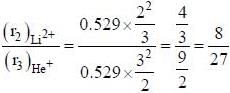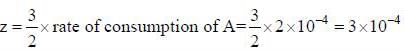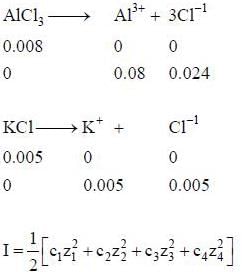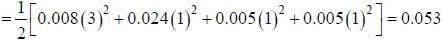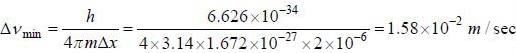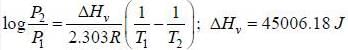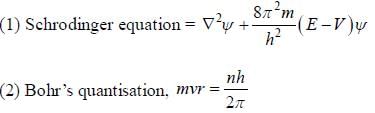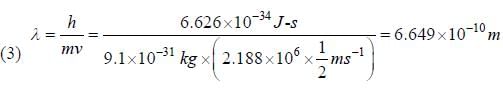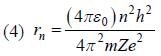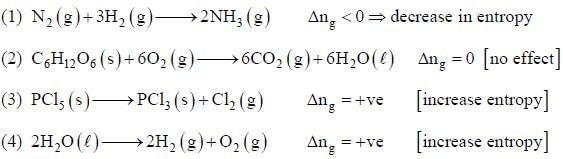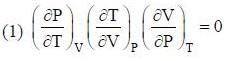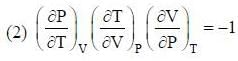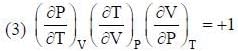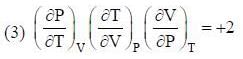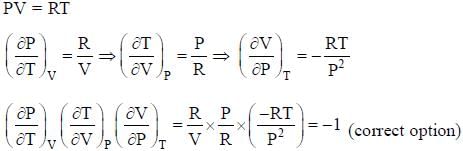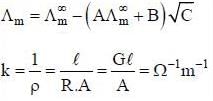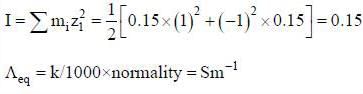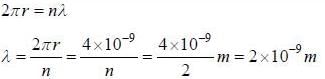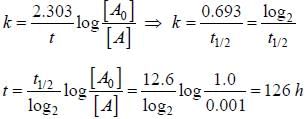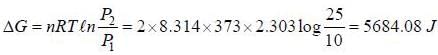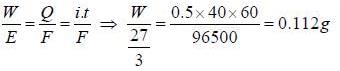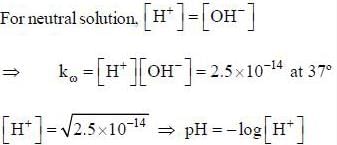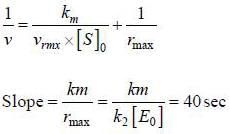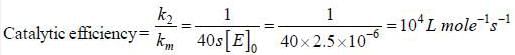Physical Chemistry MCQ - Biotechnology Engineering (BT) MCQ
21 Questions MCQ Test Mock Test Series of IIT JAM Biotechnology 2025 - Physical Chemistry MCQ
The angular momentum of an electron present in 3s and 3p orbital are
The reduction potential at 25°C for Fe3+ Fe2+ electrode if the concentration of Fe+2 ion is five times that of Fe+3 ion 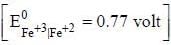
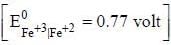
The rate constant of a reaction is 1.5 x 107 s-1 at 50°C and 4.5 x 107 s-1 at 100°C. The Arrehenius parameter A and εa is
The value AH of the reaction.
CH2Cl2(g) → C(g)+2H(g) + 2Cl(g)
The average bond energies of C-H and C-Cl bonds are 416 and 328 kJ mol respectively
Certain mass of a gas occupy 200 mL at 127°C. If the gas is cooled to -73°C at constant pressure, its new volume is
The ratio of the radius of second orbit of Li2+ ion with the radius of third orbit of He+ ion is
For a reaction. 2A + B → 3Z if the rate consumption of A is 2 x 10-4 mol dm-3 s-1. The rate of formation of Z (in mol dm-3 s-1) will be
The ionic strength of a solution containing 0.008 M AlCl3 and 0.005 M KCl is
The uncertainty in measuring the position of a proton is 2 x 10-6 m. The minimum uncertainty in measuring the speed of this proton is (mass of proton is 1.672 x 10-27 kg)
The vapour pressure of liquid decane is 10 torr at 55.7°C and 400 torr at 150.6°C. The value of ΔHv is
The collect statement in the following is/are
(1) the correct expression for Schrodinger’s equation is 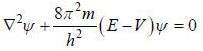
(2) Bohr's quantisation condition is mvr = nh/2π
(3) The de-Broglie wavelength of an electron revolving in the second orbit of hydrogen atom is 6.649 x 10-10m
(4) Radius of nth orbit 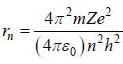
Among the following the reaction that is accompanied by a increase in the entropy is
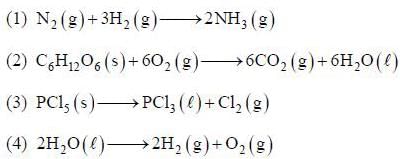
The conect equation is
Which of the following statements about catalyst is correct
(1) A catalyst increase the rate of a reaction without being destroyed
(2) A catalyst does not change the position of equilibrium
(3) A catalyst increase the rate of both forward and backward reactions
(4) A catalyst speeds up a reaction without taking pait in reaction
The collect statement is are
(1) Debye-Huckel Onsagar equation is 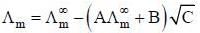
(2) The unit of specific resistance is Ω-1m-1s
(3) The ionic strength of 0.15 molal KCl solution is 0.15
(4) The unit of equivalent conductance 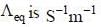
The circumference of the second orbit of an atom or ion having single electron is 4 x 10-9 m. The de-Broglie wavelength of electron revolving in this orbit is ____10-9 m
The half life of a first order reaction is 12.6 h. The time at which the concentration of reactants becomes 0.001 M from 1.0 M is _____ h.
Two moles of an ideal gas are compressed isothermally (100°C) and reversibly from a pressure of 10 atm to 25 atm. The value of free energy is ______
A current of 0.5 A is passed through molten AlCl3 for 40 min the mass of aluminium deposited at the cathode is _____ g (Al = 27)
The pH of a neutral solution at 37°C, where kω equals 2.5 x 10-14 is ______
For an enzyme substrate reaction a plot between 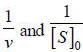 yields a slope ot 40s. It the enzyme concentration is 2.5 μM. Then the catalytic efficiency of the enzyme is 10x L mol-1s-1. The value of x is _____
yields a slope ot 40s. It the enzyme concentration is 2.5 μM. Then the catalytic efficiency of the enzyme is 10x L mol-1s-1. The value of x is _____