Practice Test - NEET MCQ
30 Questions MCQ Test 4 Months Preparation for NEET - Practice Test
electromagnetic induction i.e currents can be induced in coils (Select the best)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
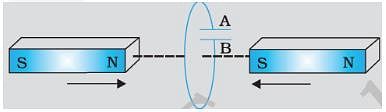
Predict the polarity of the capacitor in the situation described by fig.

An ac generator consists of 8 turns of wire, each of area A=0.0900m2 , and the total resistance of the wire is 12.0Ω. The loop rotates in a 0.500-T magnetic field at a constant frequency of 60.0 Hz. Maximum induced emf is
Heat is supplied to the gas, but its internal energy does not increase. What is the process involved?
Find the final temperature of one mole of an ideal gas at an initial temperature to t K.The gas does 9 R joules of work adiabatically. The ratio of specific heats of this gas at constant pressure and at constant volume is 4/3.
The compound formed as a result of oxidation of ethyl benzene by KMnO4 is
Propanamide on treatment with bromine in an aqueous solution of sodium hydroxide gives:
We can obtain ethylamine by Hoffmann bromamide reaction. The amide used in this reaction is:
Nitro compounds are reduced to amines. The catalyst that is preferred is:
A pure tall and a pure dwarf plant were crossed and produced offspring. Offspring were self crossed.Then find out the ratio between true breeding tall to true breeding dwarf ?
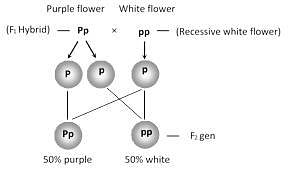
Heterozygous purple flower is crossed with recessive white flower. The progeny has the ratio:
Mating of an organism to a double recessive in order to determine whether it is homozygous or heterozygous for a character under consideration is called
|
514 videos|1455 docs|646 tests
|
|
514 videos|1455 docs|646 tests
|


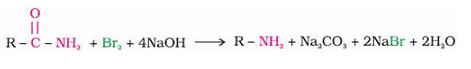
 is a tertiary amine having IUPAC name as:
is a tertiary amine having IUPAC name as: