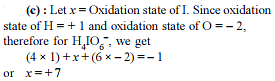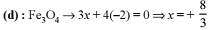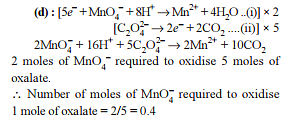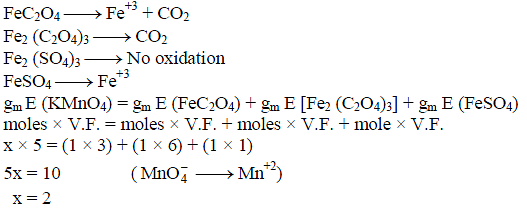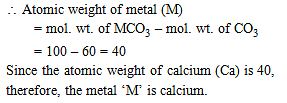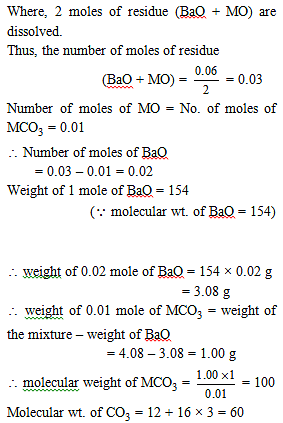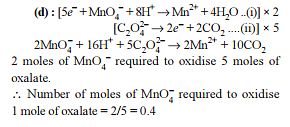Redox Reaction - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Redox Reaction - 1
When a zinc rod is kept in a copper nitrate solution what happens?
Which of the following electrodes will act as anodes, when connected to Standard Hydrogen Electrode?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the reaction of metallic cobalt placed in nickel sulphate solution, therein is a competition for release of electrons At equilibrium, chemical tests reveal that both Ni+2 (aq) and Co+2 (aq) are present at moderate concentrations. The result is that:
Given balanced chemical equation for oxidation of phosphorus (III) sulfide by nitric acid. The products include NO and SO2. Find the value of a,b,c,d,e in the following equation.
a P4S6 + 44H+ + b NO3– → cNO + dH3PO4 + e SO2 + 4H2O
Write your answer as (a + b + c + d + e)
In order to oxidise a mixture of one mole of each of FeC2O4, Fe2(C2O4)3, FeSO4 and Fe2(SO4)3 in acidic medium, the number of moles of KMnO4 required is -
4.08 g of a mixture of BaO and unknown carbonate MCO3 was heated strongly. The residue weighed 3.64 g. This was dissolved in 100 mL of 1 M HCl. The excess acid required 16 mL of 2.5 M NaOH solution for complete neutralization. Identify the metal M
How many grams of copper will be replaced in 2 L of a 1.50-M CuSO4 solution if the latter is made to react with 27.0 g of aluminium ?
(Cu = 63.5, Al =27.0)
(3CuSO4 + 2Al ® Al2(SO4)3 + 3Cu)
In order to oxidise a mixture of one mole of each of FeC2O4, Fe2(C2O4)3, FeSO4 and Fe2(SO4)3 in acidic medium, the number of moles of KMnO4 required is -
4.08 g of a mixture of BaO and unknown carbonate MCO3 was heated strongly. The residue weighed 3.64 g. This was dissolved in 100 mL of 1 M HCl. The excess acid required 16 mL of 2.5 M NaOH solution for complete neutralization. Identify the metal M
A sample of Na2CO3. H2O weighing 1.24 g is added to 200 mL of a 0.1-N H2SO4 solution. The resulting solution becomes -
One mole of a mixture of CO and CO2 requires exactly 20 gram of NaOH in solution for complete conversion of all the CO2 into Na2CO3. How many grams more
of NaOH would it require for conversion into Na2CO3 if the mixture (one mole) is completely oxidised to CO2 ?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|