Science Olympiad Test: Carbon and Its Compounds- 2 - Class 10 MCQ
15 Questions MCQ Test Olympiad Preparation for Class 10 - Science Olympiad Test: Carbon and Its Compounds- 2
The correct electron dot structure of a water molecule is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Ethanol reacts with sodium and forms two products. These are
Carbon exists in the atmosphere in the form of
Which of the following is not a saturated hydrocarbon
Which one of the following is a denatured alcohol?
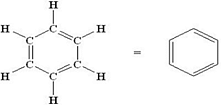
Structural formula of alkene is
Which one of the following is a functional group of alcohol?
Which of the following is the correct representation of electron dot structures of nitrogen?
Oils on treating with hydrogen in the presence of palladium or nickel catalyst from fats. This is an example of
The first member of alkyne homologous series is
While cooking if the bottom of the vessel is getting blackened on the outside. It means that
The heteroatoms present in
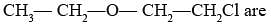
(i) Oxygen
(ii) Chlorine
(iii) Carbon
(iv) Hydrogen
|
13 videos|44 docs|187 tests
|
|
13 videos|44 docs|187 tests
|