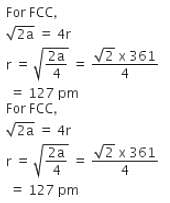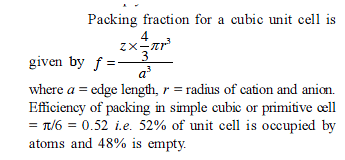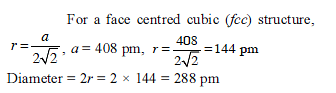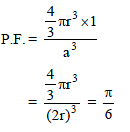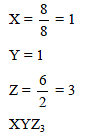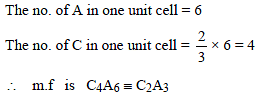Solid State - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Solid State - 1
What type of crystal defect is indicated in the diagram below ?
[AIEEE-2004]


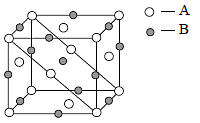
An ionic compound has a unit cell consisting of A ions at the corners of a cube and B ions on the centres of the faces of the cube. The empirical formula for this compound would be
[AIEEE-2005]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Copper crystallizes in fcc with a unit cell length of 361 pm. What is the radius of copper atom?
In a compound, atoms of element Y form ccp lattice and those of element X occupy 2/3rd of tetrahedral voids. The formula of the compound will be -
[AIEEE 2008]
Copper crystallises in fcc with a unit cell length of 361 pm. What is the radius of copper atom?
If 'a' stands for the edge length of the cubic systems : simple cubic, body centred cubic and face centred cubic, then the ratio of radii of the spheres in these system will be respectively -
What is the diameter of the largest sphere that will fit in the void at the centre of the cube edge of a BCC crystal of edge length a ?
The packing fraction of the element that crystallizes in simple cubic arrangement is -
A solid compound contains X, Y and Z atoms in a cubic lattice with X atoms occupying the corners, Y atoms in the body centred positions and Z atoms at the centre of the faces of the unit cell. What is the empirical formula of the compound ?
When heated above 916ºC, iron changes its bcc crystalline form to fcc without the change in the radius of atom. The ratio of density of the crystal before heating and after heating is [At . wt. Fe = 56]
The anions (A) form hexagonal closest packing and the cations (C) occupy only 2/3 of octahedral holes. The simplest formula of the ionic compound is –
A crystal is made of particles A and B. A forms FCC packing and B occupies all the octahedral voids. If all the particles along the plane as shown in figure are removed, then, the formula of the crystal would be :
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|