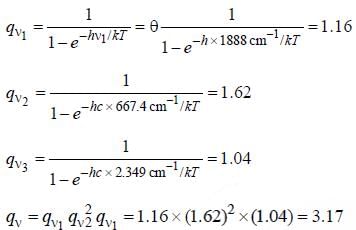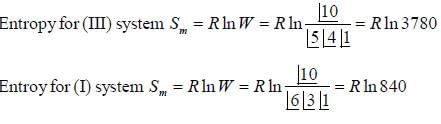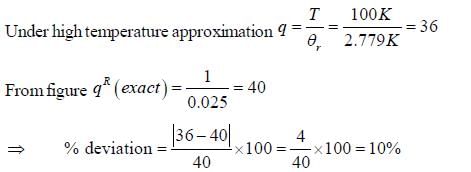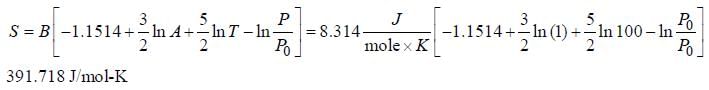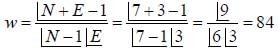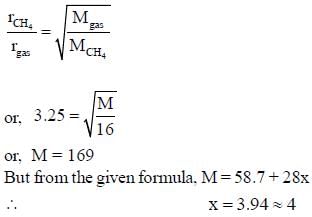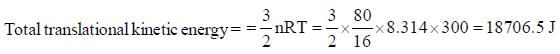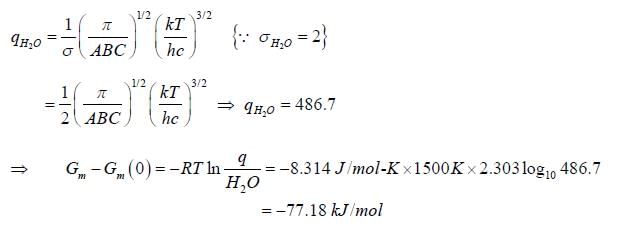Statistical Therm & Theory Of Gases - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Statistical Therm & Theory Of Gases
CO2 has the following vibrational degree of freedom 1388, 667.4(doubly degenerate) and 2349cm-1. The value of total vibrational partition function for this molecule at 1000K is ____(Round off to two decimal places).
Imagine gaseous Ar at 298k confined to move in a two dimensional plane of area 1.00 cm2. The value of partition funciton is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
For which of the following molecule is the high temperature expression for the rotational partition function valid if T = 40K is
The value of rotational partition function for the following species at 298 K is
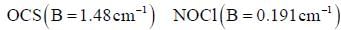
Four identical fermion particles are to be distributed in two energy levels E1 and E2. Energy E1 is 5-fold degenerate and E2 is 6-fold degenerate. The energy corresponding to the distribution having maximum number of arrangements is
The correct form of vibrational partition function is
The value of temperature at which 10% of the molecule in a system will be in first excited electronic state if this state is 400 kJ/mol above ground state is
The ground state of Co2+ ion in CoSO4.7H2O may be regarded as 4T3/2. The entropy of the solid at temperature below 1K is derived almost entirely from electron spin. The molar entropy of the solid at these temperature is ________J/mol-K.(Round off to two decimal places).
Consider following energy levels with given number of particles

If system (III) is undergoing cooling, change in molar entropy of the system is _______J/mole-K. (Round off to one decimal place)
The exact probability distribution of rotational energy levels is the Boltzmann factor of each level Weighted by the degeneracy over the partition function
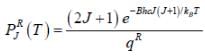
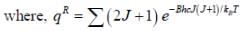
The plot of PRJ(T) vs J rotational quantum number for hetronuclear diatomic molecule at 100K is as follows
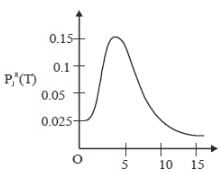
If the characteristic rotaional temperature for the molecule is 2.779K. The percentage deviation between exact rotational partition function and the partition function calculated by high-temperature approximation is ____%. (answer should be an integer).
The correct form of Sackur Tetrode Equation is
The quantity remain constant in Grand Canonical Ensemble
Molar entropy of H-atom at 1000 K and 1 bar is
Using Boltzmann porbability distribution expression. The value of possible ways of distributing 7 distingusabe particle among 4 energy levels with energies 0, ε, 2ε and 3ε respectively ____(Total energy of the system remain constant at 3ε)(answer should be an integer).
Certain mass of a gas occupy 200mL at 1270C. If the gas is cooled to -730C constant pressure, its new volume _____ mL.(an answer should be an integer).
In what mass ratio, He and O2 gases should be mixed together to have the same partial pressures is
Nickel forms a gaseous compound of the formula Ni(CO)x. The value of x given that under the same conditions of temperature and pressure methane effuses 3.25 times faster than the compound is _____(answer should be an integer). (Given : Ni = 58.7)
A vessel contains 80g methane at 270C. The total translational kinetic energy of molecules is _____kJ. (Round off to two decimal places)
The rate of effusion of an unknown gas, X, at 480K is 1.60 times the rate of effusion of sulphur dioxide gas at 300K. The molecular weight of X is ______ (answer should be an integer).
If rotational constant for H2O(g) at 15000K are A = 27.8778cm-1, B = 14.5042cm-1 and C = 9.286cm-1. The value of change in rotational free energy. (Round off to two decimal places).
[Given: Gm - Gm(0) is _____kJmol-1.
|
18 docs|37 tests
|
|
18 docs|37 tests
|