Test: Alkanes : Preperation and Projections - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Alkanes : Preperation and Projections
The end product (C) in the following sequence of reactions is


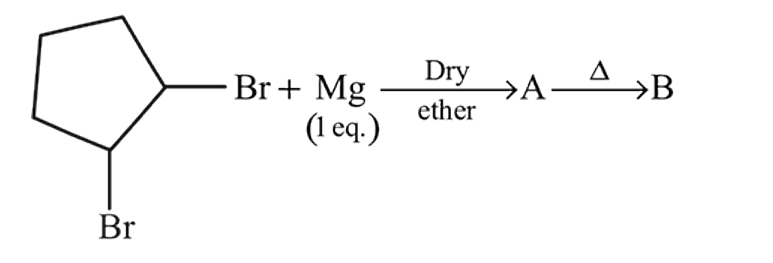
Predict the product 'B' in the sequence of reactions


| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
One mole of alkene X on ozonolysis gave one mole of acetaldehyde and one mole of acetone. The IUPAC name of X is
An alkene having molecular formula C7H14 was subjected to ozonolysis in the presence of zinc dust. An equimolar amount of the following two compounds was obtained

The IUPAC name of the alkene is
Which of the following will yield a mixture of 2-chlorobutene and 3-chlorobutene on treatment with HCl?
Which of the following statements is incorrect regarding dehydrohalogenation of alkenes?
Predict the product (A) of the following reaction

Which of the following alkenes will yield 2-methyl propanal on reductive ozonolysis. (addition with ozone followed by the reaction with Zn/H2O ).
Which one of the following compounds would have the highest heat of hydrogenation?
Which of the following types of reaction occur when a reactant has got a double bond ?
(i) Addition
(ii) Photolysis
(iii) Nucleophilic substitution
(iv) Polymerization
HOCl reacts on 3 -methyl-2-pentene, the main product will be :
In the following reaction, A and B respectively are

Ethylene can be prepared in good yield by
In the following reaction, compound (B) is
In the dehydration of,

The product formed are
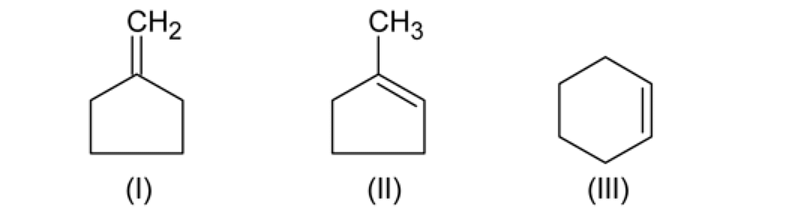
Which of the following wil have least hindered rotation about carbon carbon bond?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|




































