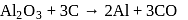Test: Aluminium and Its Compounds - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Aluminium and Its Compounds
Which of the following statement about anhydrous aluminum chloride is correct?

The incorrect statement regarding above reactions is:

The incorrect statement regarding above reactions is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the following sets of reactants which two sets best exhibit the amphoteric characters of

Set 1:  and
and  aq
aq 
Set 2: 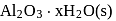 and
and 
Set 3:  and
and 
Set 4:  and
and 

Set 1:
 and
and  aq
aq 
Set 2:
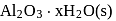 and
and 
Set 3:
 and
and 
Set 4:
 and
and 
On adding ammonium hydroxide solution to Al2(SO4)3(aq):
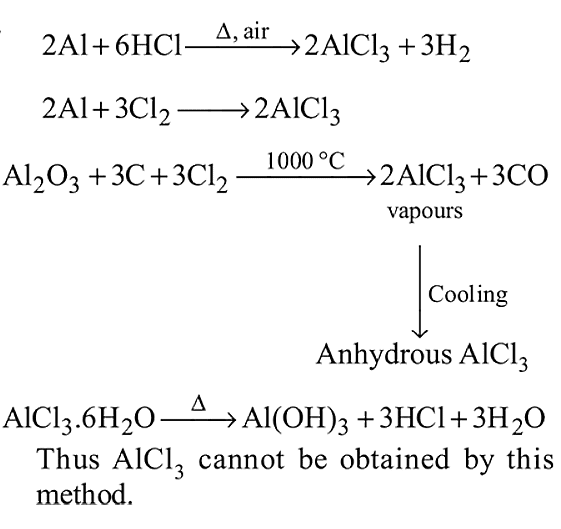
AlCl3 achieves stability by forming a dimer. In trivalent state the compound is hydrolysed in water. AlCl3 in acidified aqueous solution forms
The dissolution of Al(OH)3 by a solution of NaOH results in the formation of:
Anhydrous aluminium chloride (Al2Cl6) is covalent compound and soluble in water giving:
Anhydrous AlCl3 cannot be obtained from which of the following reactions?
In the context of the Hall-Heroult process for the extraction of Al which of the following statements is false ?
Aluminium reacts with NaOH and forms compound ' X '. If the coordination number of aluminium in ' X ' is 6 , the correct formula of X is
Which of the following properties of aluminium makes it useful for food packaging ?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|



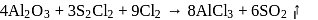




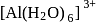 ion.
ion.

 in aqueous solution.
in aqueous solution.