JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Balancing of redox reactions, Types of redox reactions - JEE MCQ
Test: Balancing of redox reactions, Types of redox reactions - JEE MCQ
Test Description
10 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Balancing of redox reactions, Types of redox reactions
Test: Balancing of redox reactions, Types of redox reactions for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Balancing of redox reactions, Types of redox reactions questions and answers have been
prepared according to the JEE exam syllabus.The Test: Balancing of redox reactions, Types of redox reactions MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Balancing of redox reactions, Types of redox reactions below.
Solutions of Test: Balancing of redox reactions, Types of redox reactions questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Balancing of redox reactions, Types of redox reactions solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Balancing of redox reactions, Types of redox reactions | 10 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Balancing of redox reactions, Types of redox reactions - Question 1
Which of the following represents a redox reaction?
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 1
Test: Balancing of redox reactions, Types of redox reactions - Question 2
In the disproportionation reaction  , the equivalent mass of the oxidizing agent is (molar mass of
, the equivalent mass of the oxidizing agent is (molar mass of  )
)
 , the equivalent mass of the oxidizing agent is (molar mass of
, the equivalent mass of the oxidizing agent is (molar mass of  )
)
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Balancing of redox reactions, Types of redox reactions - Question 3
Which of the following is only a redox reaction but not a disproportionation reaction?
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 3
Test: Balancing of redox reactions, Types of redox reactions - Question 4
Which of the following do not show disproportionation reaction? 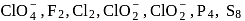 , and
, and 
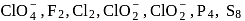 , and
, and 
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 4
Test: Balancing of redox reactions, Types of redox reactions - Question 5
Determine the oxidation number of the underlined atom in 
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 5
Test: Balancing of redox reactions, Types of redox reactions - Question 6
The oxidation state of  in
in  is
is
 in
in  is
is
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 6
Test: Balancing of redox reactions, Types of redox reactions - Question 7
Oxidation state of sulphur in anions  and
and  increases in the orders:
increases in the orders:
 and
and  increases in the orders:
increases in the orders:
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 7
Test: Balancing of redox reactions, Types of redox reactions - Question 8
Amongst the following, identify the species with an atom in  oxidation state:
oxidation state:
 oxidation state:
oxidation state:
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 8
Test: Balancing of redox reactions, Types of redox reactions - Question 9
Point out the correct statement of the following about 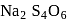
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 9
Test: Balancing of redox reactions, Types of redox reactions - Question 10
A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes maximum change in the oxidation number?
Detailed Solution for Test: Balancing of redox reactions, Types of redox reactions - Question 10
|
352 videos|596 docs|309 tests
|
Information about Test: Balancing of redox reactions, Types of redox reactions Page
In this test you can find the Exam questions for Test: Balancing of redox reactions, Types of redox reactions solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Balancing of redox reactions, Types of redox reactions, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


 is
is  in both reactant
in both reactant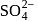 ion does not change. In option (d) is a redox reaction.
ion does not change. In option (d) is a redox reaction. (Oxidation)
(Oxidation) (Reduction)
(Reduction)

 oxidation number
oxidation number 


 involves change of oxidation state of
involves change of oxidation state of  from 0 to +3 and that of
from 0 to +3 and that of  from +4 to +2 .
from +4 to +2 . being most electronegative element cannot exhibit any positive oxidation state. In
being most electronegative element cannot exhibit any positive oxidation state. In 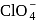 chlorine is present in its highest oxidation state i.e
chlorine is present in its highest oxidation state i.e  . Therefore it does not show disproportionation reaction.
. Therefore it does not show disproportionation reaction. be
be  , then
, then

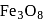












 . No. of
. No. of 



 has the structure :
has the structure :
 atoms are
atoms are  each and that of other two S atoms is zero each.
each and that of other two S atoms is zero each.
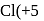 to
to 















