Test: Clausius-Clapeyron Equation - Chemistry MCQ
10 Questions MCQ Test Physical Chemistry - Test: Clausius-Clapeyron Equation
During phase transitions like vaporization, melting and sublimation
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The vapour pressure p in kPa at temperature T can be given by the relation
The slope of sublimation curve is ____ the slope of the vaporization curve at triple point.
An application requires R-12 at −140°C. The triple-point temperature is −157°C. Find the pressure of the saturated vapour at the required condition.
Estimate the freezing temperature of liquid water at a pressure of 30 MPa.
Which of the following requirement is satisfied by a phase change of the first order?
Water ____ on melting and has the fusion curve with a ____ slope.
According to Trouton’s rule, the ratio of latent heat of vaporization to the boiling point at 1.013 bar is
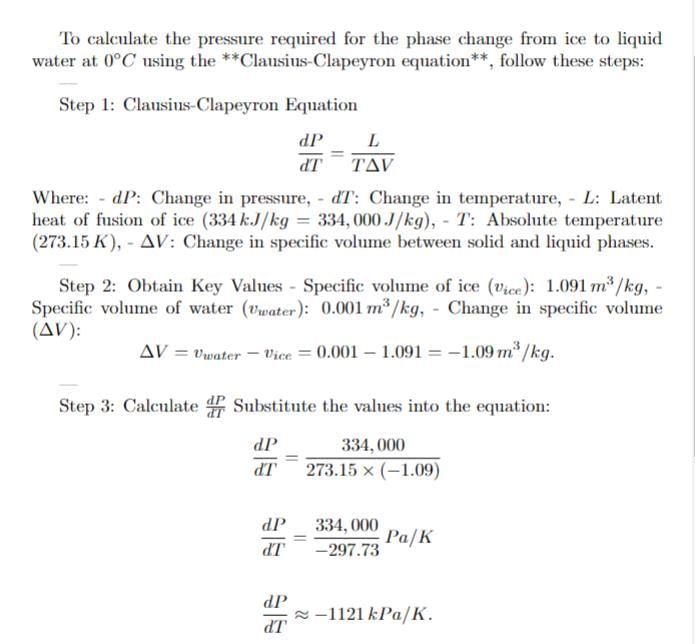
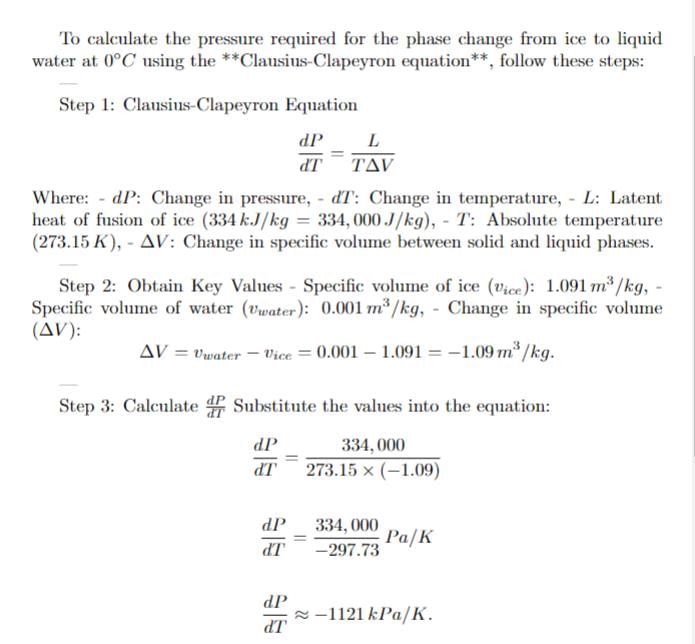
Ice (solid water) at 0 degree celcius and 100 kPa is compressed isothermally until it becomes liquid. Find the required pressure for the phase change.
|
83 videos|142 docs|67 tests
|