JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - JEE MCQ
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - JEE MCQ
Test Description
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law questions and answers have been
prepared according to the JEE exam syllabus.The Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law below.
Solutions of Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 1
A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as:
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 1
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 2
Schematic diagram of an electrolytic cell is
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 3
The metal that can not be obtained by electrolysis of an aqueous solution of its salt is
[JEE Main 2014]
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 3
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 4
A concentration cell is a galvanic cell in which
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 4
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 5
How long a current of 3 amperes has to be passed through a solution of  to coat a metal surface of
to coat a metal surface of 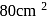 and
and  thick layer. Density of
thick layer. Density of  is
is 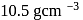
 to coat a metal surface of
to coat a metal surface of 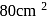 and
and  thick layer. Density of
thick layer. Density of  is
is 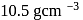
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 5
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 6
In which of the following cells, the space between cathode and anode is filled by a moist mixture of ammonium choride and zinc chloride?
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 6
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 7
Which colourless gas evolves, when 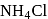 reacts with zinc in a dry cell battery
reacts with zinc in a dry cell battery
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 7
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 8
The e.m.f. of a Daniell cell at 298 K is 
When the concentration of ZnSO4 is 1.0M and that of CuSO4 is 0.01M, the e.m.f. changed to E2. What is the relationship between E1 and E2 ?
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 8
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 9
 of pure silver was converted into silver nitrate and its solution was taken in a beaker. It was electrolysed using platinum cathode and silver anode.
of pure silver was converted into silver nitrate and its solution was taken in a beaker. It was electrolysed using platinum cathode and silver anode.  Faraday of electricity was passed using
Faraday of electricity was passed using  volt above the decomposition potential of silver. The silver content of the beaker after the above shall be
volt above the decomposition potential of silver. The silver content of the beaker after the above shall be
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 9
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 10
The  acts as sacrificial or cathodic protection to prevent rusting of iron because :
acts as sacrificial or cathodic protection to prevent rusting of iron because :
 acts as sacrificial or cathodic protection to prevent rusting of iron because :
acts as sacrificial or cathodic protection to prevent rusting of iron because :
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 10
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 11
Two half cells have reduction potentials  and
and  respectively. A galvanic cell is made from these two half cells. Which of the following statements is correct?
respectively. A galvanic cell is made from these two half cells. Which of the following statements is correct?
 and
and  respectively. A galvanic cell is made from these two half cells. Which of the following statements is correct?
respectively. A galvanic cell is made from these two half cells. Which of the following statements is correct?
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 11
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 12
Aqueous solution of which of the following compounds is the best conductor of electric current?
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 12
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 13
A hydrogen gas electrode is made by dipping platinum wire in a solution of  of
of  and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of electrode would be ?
and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of electrode would be ?
 of
of  and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of electrode would be ?
and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of electrode would be ?
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 13
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 14
Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 15
In the silver plating of copper,  is used instead of
is used instead of  . The reason is
. The reason is
 is used instead of
is used instead of  . The reason is
. The reason is
Detailed Solution for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law - Question 15
|
352 videos|596 docs|309 tests
|
Information about Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law Page
In this test you can find the Exam questions for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Electrolysis & Faraday's Laws, Kohlrausch’s Law, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


 Area
Area  thickness
thickness Volume
Volume  density
density Mass of
Mass of  to be deposited
to be deposited

 seconds
seconds


 , less is the EMF Hence
, less is the EMF Hence 

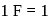 mole of electrons
mole of electrons 
 left
left 
 of
of  of
of 
 completely dissociates to give
completely dissociates to give  and
and  ions, hence act as very good electrolyte. While others are non- electrolytes.
ions, hence act as very good electrolyte. While others are non- electrolytes.




 is used instead of
is used instead of  . Copper being more electropositive readily precipitate silver from their salt solution
. Copper being more electropositive readily precipitate silver from their salt solution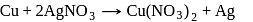
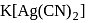 solution a complex anion
solution a complex anion  is formed and hence
is formed and hence  are less available in the solution and therefore copper cannot displace Ag from its complex ion.
are less available in the solution and therefore copper cannot displace Ag from its complex ion.














