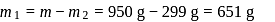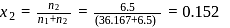Test: Empirical and molecular formulae - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Empirical and molecular formulae
A sample of AlF3 contains 3.0 × 1024 F− ions. The number of formula unit of this sample are
Calculate the density of an aqueous solution of KI, if its molarity and molality are 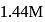 and
and  respectively.
respectively.
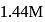 and
and  respectively.
respectively.| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What amount of conc. H2SO4 solution should be used to prepare 500 mL of 0.5MH2SO4? (The concentration of H2SO4 solution being used is 90% and molecular mass of H2SO4 = 98.079 g mol−1)
How many of  are required to react completely with
are required to react completely with  mixture of
mixture of  and
and  containing equimolar amounts of two?
containing equimolar amounts of two?
 of
of  is mixed with
is mixed with  of
of  N NaOH solution. The resulting solution is
N NaOH solution. The resulting solution is
What is the molarity of  ion in aqueous solution that contain
ion in aqueous solution that contain 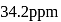 of
of 
(Assume complete dissociation and density of solution 1 g/mL)
 of a sodium carbonate solution contains
of a sodium carbonate solution contains  of
of  . If
. If  of this solution is diluted to one litre, what is the concentration of the resultant solution? (mol. wt. of
of this solution is diluted to one litre, what is the concentration of the resultant solution? (mol. wt. of  )
)
A carbon compound contains  of carbon,
of carbon,  of hydrogen and
of hydrogen and  of bromine. The molecular weight of the compound is 187.9. Calculate the molecular formula of the compound.
of bromine. The molecular weight of the compound is 187.9. Calculate the molecular formula of the compound.
(Atomic weight: H = 1.008,C = 12.0, Br = 79.9)
0.5 g of fuming  (Oleum) is diluted with water. This solution is completely neutralized by 26.7 mL of 0.4 N NaOH. The percentage of free
(Oleum) is diluted with water. This solution is completely neutralized by 26.7 mL of 0.4 N NaOH. The percentage of free  in the sample is
in the sample is
To  of
of  solution
solution 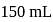 of water is added. What is the molarity of resultant solution?
of water is added. What is the molarity of resultant solution?
RH 2 (ion exchange resin) can replace Ca 2 + ions in hard water as:
RH 2 + Ca 2 + → RCa + 2H+
If 1 L of hard water after passing through RH 2 has pH = 3, then hardness in parts per million of Ca 2 + is :
If the density of 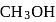 is
is  , the volume of methanol to prepare
, the volume of methanol to prepare  of
of  aqueous solution is
aqueous solution is
The concentration of ethanol in the solution called 86-proof vodka is  . If the density of the solution is
. If the density of the solution is 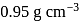 , the amount fraction of ethanol in vodka is
, the amount fraction of ethanol in vodka is
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


 the number of
the number of  is 3 for one
is 3 for one  molecule
molecule  formula unit of
formula unit of 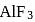
 units
units


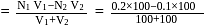
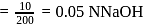








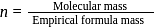


 solution is
solution is
 solution
solution 
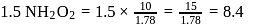
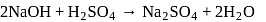
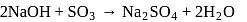
 of
of 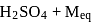 of
of  of NaOH
of NaOH

 .
.
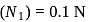

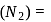 To find
To find
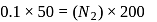

 for
for 
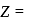 total positive charge in
total positive charge in  )
) (normality)
(normality)  (where,
(where,  is molarity).
is molarity).
 10 - 4 moles per litre
10 - 4 moles per litre 10 - 1 moles of Ca 2 + per 10 3 L
10 - 1 moles of Ca 2 + per 10 3 L 10 - 1
10 - 1  40 g Ca 2 + per 10 6 g of H 2 O
40 g Ca 2 + per 10 6 g of H 2 O , we have Amount of
, we have Amount of  required,
required, 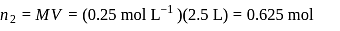
 required,
required, 
 required,
required, 
 of
of  and
and  of
of  are mixed
are mixed

 of vodka, we have
of vodka, we have  and
and  Mass of solution,
Mass of solution,