JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Equivalence concept, Redox titrations - JEE MCQ
Test: Equivalence concept, Redox titrations - JEE MCQ
Test Description
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Equivalence concept, Redox titrations
Test: Equivalence concept, Redox titrations for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Equivalence concept, Redox titrations questions and answers have been
prepared according to the JEE exam syllabus.The Test: Equivalence concept, Redox titrations MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Equivalence concept, Redox titrations below.
Solutions of Test: Equivalence concept, Redox titrations questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Equivalence concept, Redox titrations solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Equivalence concept, Redox titrations | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Equivalence concept, Redox titrations - Question 1
The mass of  produced when excess of
produced when excess of  is bubbled through a solution of
is bubbled through a solution of  is
is
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 1
Test: Equivalence concept, Redox titrations - Question 2
The ratio of amounts of  needed to precipitate all the metal ions from
needed to precipitate all the metal ions from  of
of 
 and
and  of
of 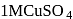 will be
will be
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Equivalence concept, Redox titrations - Question 3
0.45 g of acid (molecular weight 90) is neutralised by 20ml of 0.5 N caustic potash. The basicity of acid is
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 3
Test: Equivalence concept, Redox titrations - Question 4
 platinic chloride of a mono- acidic base on ignition gives
platinic chloride of a mono- acidic base on ignition gives  platinum. The molecular weight of the base is molecular weight of
platinum. The molecular weight of the base is molecular weight of 
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 4
Test: Equivalence concept, Redox titrations - Question 5
Given : 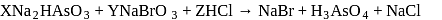
The values of 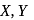 and
and 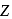 in the above redox reaction are respectively :
in the above redox reaction are respectively :
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 5
Test: Equivalence concept, Redox titrations - Question 6
The number of electrons involved in the reduction of one nitrate ion to hydrazine is
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 6
Test: Equivalence concept, Redox titrations - Question 7
The concentration of oxalic acid is ' 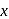 '
' 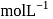 .
. 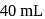 of this solution reacts with
of this solution reacts with  of
of 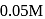 acidified
acidified  . What is the
. What is the 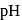 of '
of ' 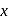 '
'  oxalic acid solution ? (Assume that oxalic acid dissociates completely)
oxalic acid solution ? (Assume that oxalic acid dissociates completely)
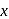 '
' 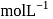 .
. 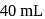 of this solution reacts with
of this solution reacts with  of
of 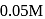 acidified
acidified  . What is the
. What is the 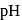 of '
of ' 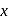 '
'  oxalic acid solution ? (Assume that oxalic acid dissociates completely)
oxalic acid solution ? (Assume that oxalic acid dissociates completely)
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 7
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 8
Test: Equivalence concept, Redox titrations - Question 9
Which of the following involves transfer of five electrons ?
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 9
Test: Equivalence concept, Redox titrations - Question 10
The colour of phenolphthalein in acid and base ranges of the indicator respectively, are
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 10
Test: Equivalence concept, Redox titrations - Question 11
Which of the following indicators can be used to detect end point in the titration of a weak acid versus strong base?
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 11
Test: Equivalence concept, Redox titrations - Question 12
The titration of a weak base versus weak acid cannot be carried out by using acid-base indicator because
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 12
Test: Equivalence concept, Redox titrations - Question 13
Which of the following indicators (buffer range provided in brackets) cannot be used to detect end point of titration between strong acid and strong base?
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 13
Test: Equivalence concept, Redox titrations - Question 14
Find the value of 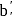 for the following half equation.
for the following half equation.

Detailed Solution for Test: Equivalence concept, Redox titrations - Question 14
Test: Equivalence concept, Redox titrations - Question 15
The product of oxidation of  with
with  in alkaline medium is:-
in alkaline medium is:-
 with
with  in alkaline medium is:-
in alkaline medium is:-
Detailed Solution for Test: Equivalence concept, Redox titrations - Question 15
|
352 videos|596 docs|309 tests
|
Information about Test: Equivalence concept, Redox titrations Page
In this test you can find the Exam questions for Test: Equivalence concept, Redox titrations solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Equivalence concept, Redox titrations, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF




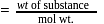
 1 mole of
1 mole of  gives 1 mole of
gives 1 mole of 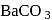
 205 mole of
205 mole of  will give 205 mole of
will give 205 mole of 
 wt. of
wt. of  mole of
mole of  will be
will be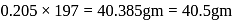

 mole
mole  mole
mole 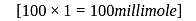
 millimole
millimole  millimole
millimole  required
required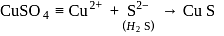
 mole
mole  millimole
millimole 
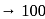 milimole
milimole  required
required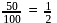
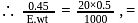 E.
E. 




 number of electrons required is 7 .
number of electrons required is 7 .





 eq. wt.
eq. wt.  mol. wt. of oxalic acid
mol. wt. of oxalic acid

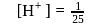

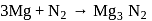
 is changing from 0 to
is changing from 0 to  , while in nitrogen it is changing from 0 to
, while in nitrogen it is changing from 0 to  . So oxidation of
. So oxidation of  and reduction of nitrogen takes place.
and reduction of nitrogen takes place. in
in  is
is 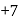 and in
and in  it is
it is  The difference is of 5 electrons
The difference is of 5 electrons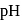 at the equivalence point is more that 7 due to hydrolysis of congugate base of the weak acid. The steep rise in
at the equivalence point is more that 7 due to hydrolysis of congugate base of the weak acid. The steep rise in  around equivalence point encompasses the range 7 to 10 . Thus, thymolphthalein can be used as the indicator to locate end point.
around equivalence point encompasses the range 7 to 10 . Thus, thymolphthalein can be used as the indicator to locate end point.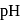 is given by
is given by
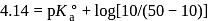
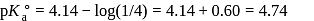
 of
of  solution, we will have
solution, we will have
 near the equivalence point.
near the equivalence point.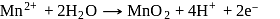
 is
is  .
.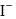 with
with  in alkaline solution is given by
in alkaline solution is given by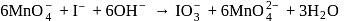
 reduced from +7 to +6 and the oxidation state of I oxidised from -1 to +5.
reduced from +7 to +6 and the oxidation state of I oxidised from -1 to +5. is involved in the reaction.
is involved in the reaction.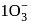 .
.














