Test: General Characteristics of Alkali Metals - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: General Characteristics of Alkali Metals
Sodium carbonate solution in water is alkaline due to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When a crystal of caustic soda is exposed to air, a liquid layer is deposited because :
The pair of compounds which cannot exits together in solution is
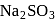 and
and 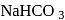 may be distinguished by treating their aqueous solution with :
may be distinguished by treating their aqueous solution with :
When sulphur is heated with 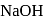 (aq). The compounds formed are
(aq). The compounds formed are
Acidified solution of sodium thiosulphate is unstable because in thiosulphate
For which one of the following minerals, the composition given is incorrect?
If 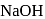 is added to an aqueous solution of
is added to an aqueous solution of 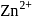 ions, a white precipitate appears and on adding excess
ions, a white precipitate appears and on adding excess 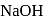 , the precipitate dissolves. In this solution zinc exists in the :
, the precipitate dissolves. In this solution zinc exists in the :
Which of the following does not form an oxide on heating?
Which of the following statements regarding alkali metals is not correct?
Which of the following sulphates has the highest solubility?
Which of the following statements is not correct?
Which of the following orders regarding the melting point of alkali chlorides is true?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


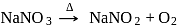
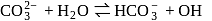
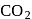 to form
to form 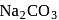
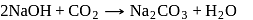
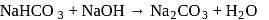
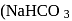 is an acidic salt)
is an acidic salt)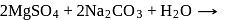
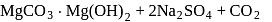
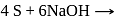
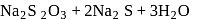
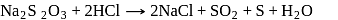
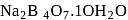 .
.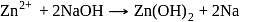
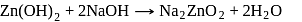
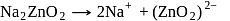
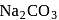 does not decompose to form
does not decompose to form 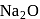 .
.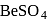 is most soluble because hydration energy is more than lattice energy
is most soluble because hydration energy is more than lattice energy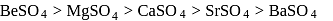
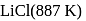 as compared to
as compared to 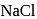 is probably because
is probably because 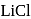 is covalent in nature and
is covalent in nature and 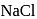 is ionic.
is ionic.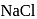 .
.














