Test: Ionic Bond and properties - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Ionic Bond and properties
Which one of the following molecules contain both ionic and covalent bonds?
In  , the formal charge on each oxygen atom and the
, the formal charge on each oxygen atom and the  -
-  bond order respectively are
bond order respectively are
 , the formal charge on each oxygen atom and the
, the formal charge on each oxygen atom and the  -
-  bond order respectively are
bond order respectively are| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following is a favourable factor for cation formation?
 and
and  have the same crystal structure and approximately the same ionic radii. If
have the same crystal structure and approximately the same ionic radii. If  is the lattice energy of
is the lattice energy of  , the approximate lattice energy of
, the approximate lattice energy of  is
is
The compound which contains both ionic and covalent bonds is
The electronegativity difference between 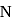 and
and  is greater than that between
is greater than that between  and
and 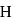 yet the dipole moment of
yet the dipole moment of  is larger than that of
is larger than that of  . This is because
. This is because
The dipole moments of diatomic molecules 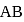 and
and  are
are 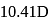 and
and  , respectively while their bond distances are
, respectively while their bond distances are 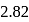 and
and  , respectively. This indicates that
, respectively. This indicates that
 and
and  respectively, what is the per cent ionic character of the bond?
respectively, what is the per cent ionic character of the bond?
The bond length of HCl molecule is 1.275Ã… and its dipole moment is 1.03D. The ionic character of the molecule (in percent) (charge of the electron = 4.8 × 10−10 esu) is
What is the % ionic character of HCl if its observed dipole moment is 1.03D, electronic charge (q) is 4.8 × 10−10 e.s.u. and distance (d) between atoms is 1.27Å ?
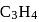 ?
?
Amongst LiCl, RbCl, BeCl2 and MgCl2 the compounds with the greatest and the least ionic character, respectively are:
 is
is 
 . The dipole moment of
. The dipole moment of 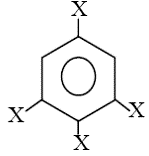 is
is
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|





 atoms. Formal charge
atoms. Formal charge 




 the atomic dipole (orbital dipole due to lone pair) and bond dipole are in the same direction whereas in
the atomic dipole (orbital dipole due to lone pair) and bond dipole are in the same direction whereas in 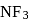 these are in opposite direction so in the former case they are added up whereas in the latter case net result is reduction of dipole moment. It has been shown in the following figure :
these are in opposite direction so in the former case they are added up whereas in the latter case net result is reduction of dipole moment. It has been shown in the following figure :
 electric charge
electric charge  bond length
bond length

 then
then  ionic character in
ionic character in 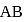

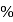 ionic character in
ionic character in 
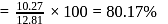
 experimental
experimental 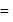 Dipole moment
Dipole moment 
 theoretical
theoretical 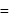 Bond length
Bond length 

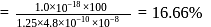



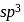 hybridisation forming four
hybridisation forming four  hybrid orbitals.
hybrid orbitals.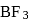 show maximum covalent character due to back bonding.
show maximum covalent character due to back bonding.
 trihalobenzene is zero due to symmetry, thus its net dipole moment is same as halo benzene.
trihalobenzene is zero due to symmetry, thus its net dipole moment is same as halo benzene.














