Test: Ionic structure, Electrical and Magnetic properties - JEE MCQ
10 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Ionic structure, Electrical and Magnetic properties
KBr has rock salt type structural arrangements and has a density of 3.70 g/cm3. The edge length of the unit cell is approximately [molecular weight of KBr = 120 g/mol]
The radii of  and
and  ions are
ions are  and 181 pm respectively. The edge length of
and 181 pm respectively. The edge length of  unit cell is
unit cell is
 and
and  ions are
ions are  and 181 pm respectively. The edge length of
and 181 pm respectively. The edge length of  unit cell is
unit cell is| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
CsBr has bcc structure with edge length 4.3. The shortest interionic distance in between Cs+ and Br− is
 crystal is correct?
crystal is correct?
 type structure, the co-ordination number of
type structure, the co-ordination number of  and
and  respectively are
respectively are
CsBr crystallises in a body centered cubic lattice. The unit cell length is 436.6pm. Given that the atomic mass of Cs = 133 and that of Br = 80amu and Avogadro number being 6.02 × 1023 mol−1 the density of CsBr is
How many unit cells are present in 4.0gm of crystal AB (formula mass of AB = 40) having rock salt type structure? (NA = Avogadro's no.)
 ratio of
ratio of  is
is  . Pick out the incorrect statements from the following?
. Pick out the incorrect statements from the following?
 , if
, if 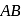 is just like the rock salt like structure then,
is just like the rock salt like structure then,  and
and  are located at
are located at 
A molecule A2 B(Mwt. = 166.4) occupies triclinic lattice with a 5Å,b = 8Å, and c = 4Å If the density of AB2 is 5.2 g cm−3, the number of molecules present in one unit cell is
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|



 lattice, the distance between the cation and anion is equal to the sum of their radii, which is equal to half of the edge length of unit cell,
lattice, the distance between the cation and anion is equal to the sum of their radii, which is equal to half of the edge length of unit cell,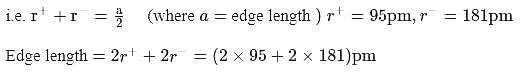



 half the distance between two nearest neighbouring atoms.
half the distance between two nearest neighbouring atoms.
 has cubic close packed
has cubic close packed 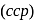 structure. The
structure. The  ions are present at the corners of the cube and at the centre of each face. Zinc ions occupy half of the tetrahedral sites. Each zinc ion is surrounded by four sulphide ions which are disposed towards the corners of a regular tetrahedron. Similarly,
ions are present at the corners of the cube and at the centre of each face. Zinc ions occupy half of the tetrahedral sites. Each zinc ion is surrounded by four sulphide ions which are disposed towards the corners of a regular tetrahedron. Similarly, 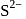 ion is surrounded by four
ion is surrounded by four 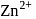 ions.
ions.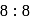 for
for 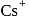 and
and 
 two formula unit of
two formula unit of  in 1 cubic unit
in 1 cubic unit  and edge length,
and edge length, 

 .
.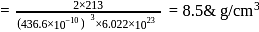
 are present in a unit cell
are present in a unit cell
 ion is too small to fit into the octahedral voids of
ion is too small to fit into the octahedral voids of  ions.
ions. is just like
is just like  . Thus twelve
. Thus twelve  are at edges and 1 within body of
are at edges and 1 within body of  i.e. in octahedral voids and
i.e. in octahedral voids and  at faces and 8 at corner.
at faces and 8 at corner.











