Chemistry Exam > Chemistry Tests > Inorganic Chemistry > Test: Magnetism - Chemistry MCQ
Test: Magnetism - Chemistry MCQ
Test Description
15 Questions MCQ Test Inorganic Chemistry - Test: Magnetism
Test: Magnetism for Chemistry 2024 is part of Inorganic Chemistry preparation. The Test: Magnetism questions and answers have been
prepared according to the Chemistry exam syllabus.The Test: Magnetism MCQs are made for Chemistry 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Magnetism below.
Solutions of Test: Magnetism questions in English are available as part of our Inorganic Chemistry for Chemistry & Test: Magnetism solutions in
Hindi for Inorganic Chemistry course. Download more important topics, notes, lectures and mock
test series for Chemistry Exam by signing up for free. Attempt Test: Magnetism | 15 questions in 30 minutes | Mock test for Chemistry preparation | Free important questions MCQ to study Inorganic Chemistry for Chemistry Exam | Download free PDF with solutions
Detailed Solution for Test: Magnetism - Question 1
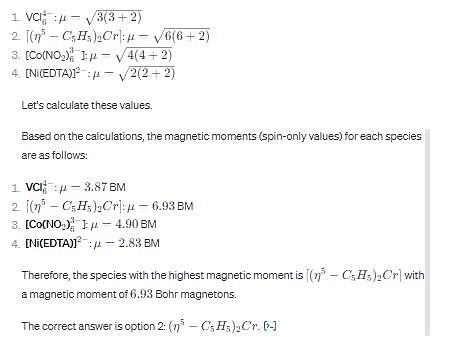
Detailed Solution for Test: Magnetism - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Magnetism - Question 3
The actual magnetic moment shows a large deviation from the spin-only formula in case of
Detailed Solution for Test: Magnetism - Question 4
Test: Magnetism - Question 5
The experimental magnetic moment of K3[Fe(CN)6] is 2.3 μB and is attributed o the
Test: Magnetism - Question 10
The plot of xT versus T data of an ideal paramagnetic sample will
Test: Magnetism - Question 12
In tetrahedral geometry, which one of the following sets of electronic-configuration will have orbital contribution to the magnetic moment?
Test: Magnetism - Question 13
The magnetic moment of an octahedral Co(II) complex is 4.0 uB. The d-electron configuration of Co(II) is:
Test: Magnetism - Question 15
The metal ion which is most likely to show the low spin-high spin equilibria in its complexes has the electronic configuration
|
48 videos|92 docs|41 tests
|
Information about Test: Magnetism Page
In this test you can find the Exam questions for Test: Magnetism solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Magnetism, EduRev gives you an ample number of Online tests for practice