Test: Minerals and ores - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Minerals and ores
Which of the following statement is not correct about Ellingham diagram?
Which of the ore dressing process requires finest size of ore?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which among the following is used as flux in the extraction of iron from oxide ores?
Which of the following is not added during the extraction of silver by cyanide process?
The following reactions take place in the blast furnace in the preparation of impure iron. Identify the reaction pertaining to the formation of the slag.
Aluminium is extracted from alumina 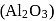 by electrolysis of a molten mixture of
by electrolysis of a molten mixture of
Consider the following metallurgical processes:
(I) Heating impure metal CO and distilling the resulting volatile carbonyl (b.p. 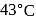 ) and finally decomposition at
) and finally decomposition at  to get the pure metal
to get the pure metal
(II) Heating the sulphide ore in air until a part is converted to oxide and then furher heating in the absence of air to let the oxide react with unchanged metal sulphide
(III) Electrolysis of the molten electrolyte containing approximately equal amounts of the metal chloride and  to obtained the metal The processes used for obtaning magnesium, nickel and copper are respectively:
to obtained the metal The processes used for obtaning magnesium, nickel and copper are respectively:
When copper pyrites is roasted in excess of air, a mixture of  is formed.
is formed. 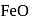 is present as impurity. This can be removed as slag during reduction of
is present as impurity. This can be removed as slag during reduction of  . The flux added to form slag is
. The flux added to form slag is
What is the role of limestone during the extraction of iron from haematite ore?
In the extraction of aluminium by Hall-Heroult process, purified  is mixed with
is mixed with  to
to
(i) lower the melting point of  .
.
(ii) increase the conductivity of molten mixture.
(iii) reduce 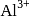 into
into 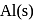 .
.
(iv) acts as catalyst.
In Hall-Heroult process how much carbon anode is burnt away to produce each  of aluminium?
of aluminium?
Which statement is incorrect regarding Hall-Heroult process for metallurgy of aluminium?
1. Use of 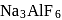 lowers the melting point of
lowers the melting point of 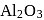
2. Steel vessel with lining of carbon acts as cathode.
3. Graphite is used as anode.
4. Carbon dioxide gas is generated at cathode.
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|



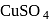 is electrolysed using platinum electrodes,
is electrolysed using platinum electrodes,
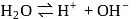


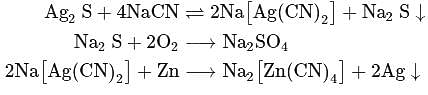
 is cryolite and used in the electrolysis of alumina to lower the melting point and increase electrical conductivity.
is cryolite and used in the electrolysis of alumina to lower the melting point and increase electrical conductivity. , calcium carbonate is almost completely decomposed to give
, calcium carbonate is almost completely decomposed to give  which acts as a flux and combines with
which acts as a flux and combines with  present as impurity (gangue) in the ore to form calcium silicate (fusible slag)
present as impurity (gangue) in the ore to form calcium silicate (fusible slag) (acidic flux)
(acidic flux)  is a bad conductor of electricity. Therefore, cryolite
is a bad conductor of electricity. Therefore, cryolite  and fluorspar
and fluorspar 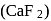 are added to purified alumina which not onlymake alumina a good conductor of electricity but also reduce the melting point of the mixture to around
are added to purified alumina which not onlymake alumina a good conductor of electricity but also reduce the melting point of the mixture to around 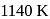 .
.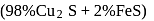
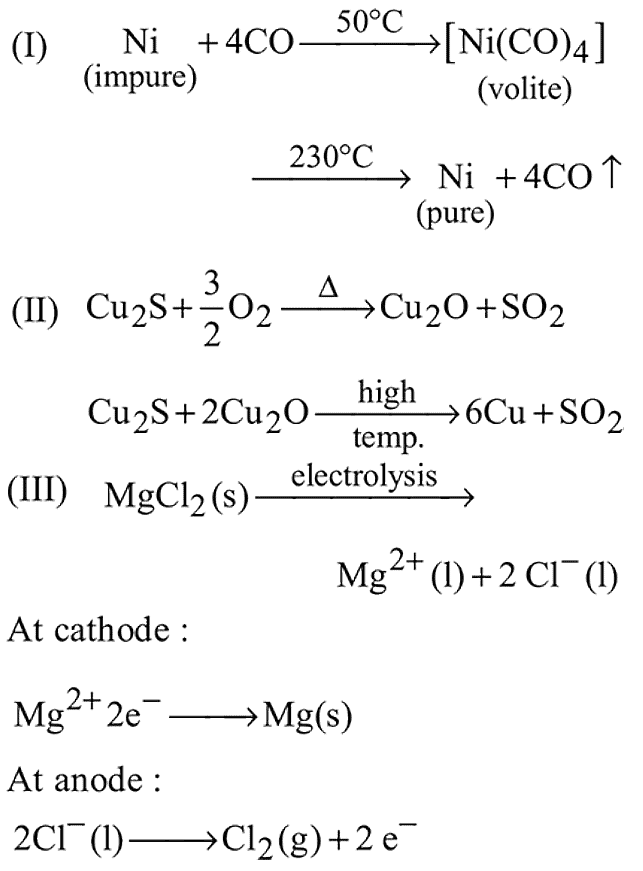
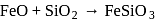
 , spongy iron is obtained. In slag zone
, spongy iron is obtained. In slag zone  , limestone reacts with impurity (e.g., silica) to form slag. In the combustion and fusion zone
, limestone reacts with impurity (e.g., silica) to form slag. In the combustion and fusion zone  , all the charge melts and pig iron is obtained which purified further.
, all the charge melts and pig iron is obtained which purified further. of
of  produced, about
produced, about 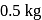 of carbon anode is burnt away.
of carbon anode is burnt away.














