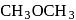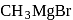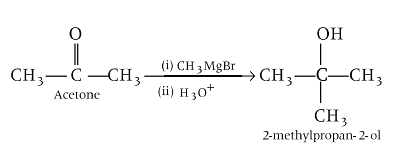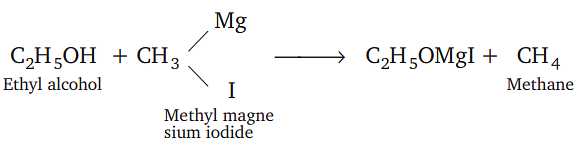Test: Preparation and Properties of Alcohols - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Preparation and Properties of Alcohols
How many structural isomers of 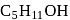 will be primary alcohols?
will be primary alcohols?
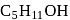 will be primary alcohols?
will be primary alcohols?Which of the following species can act as the strongest base?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
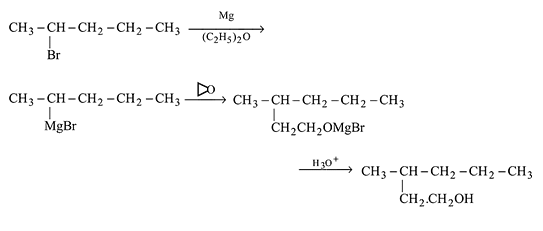
Which of the following synthesis gives 3 -methyl-1- hexanol?
Which of the following are intermediates in the reaction of excess of 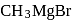 with
with 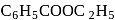 to make 2 -phenyl
to make 2 -phenyl 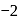 -propanol?
-propanol?
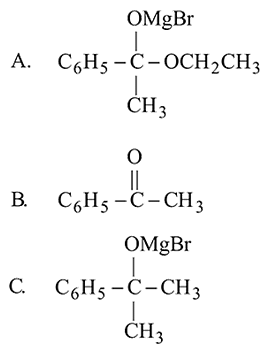
Which one/ones of the following reactions will yield 2-propanol?
I. 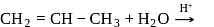
II.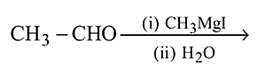
III.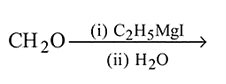
IV. 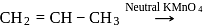
Which of the following has lowest boiling point?
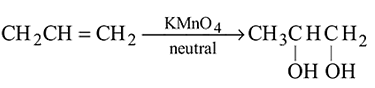
The following change can be carried out with
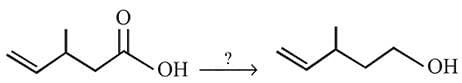
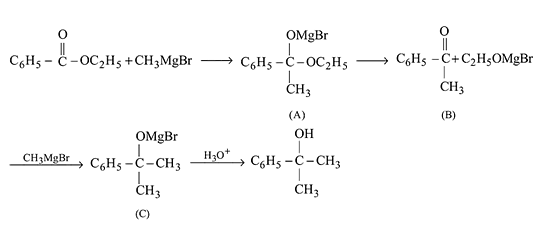
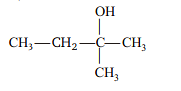
Ethyl magnesium bromide reacts with acetone to give X. On hydrolysis X forms
Identify the product [Z] in the given sequence of reaction
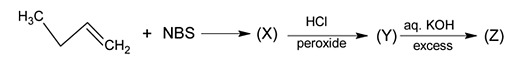

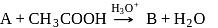
In the above reactions ' 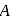 ' and '
' and ' 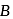 ' respectively are
' respectively are
The boiling points of isomeric alcohols follow the order
Identify the suitable reagent for the reaction given below.
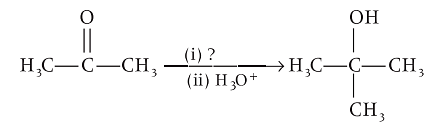
Which one of the following gases is liberated when ethyl alcohol is heated with methyl magnesium iodide?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


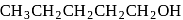
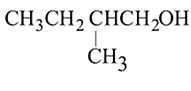
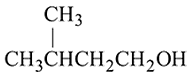
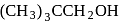
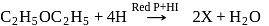 ;
;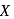 is
is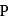 and HI to alkanes through alkyl iodides
and HI to alkanes through alkyl iodides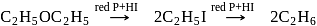
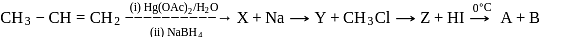
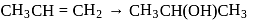
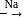
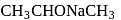
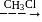
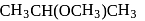
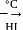

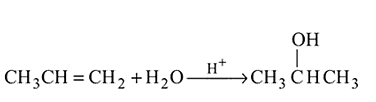
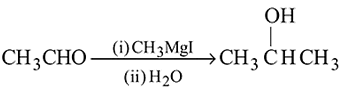
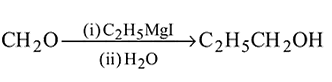
 -Nitrophenol has intramolecular H-bonding.
-Nitrophenol has intramolecular H-bonding. alcohols using Lithium aluminum hydride
alcohols using Lithium aluminum hydride 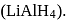 An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid.
An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid.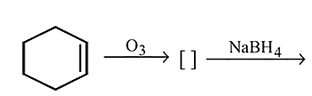
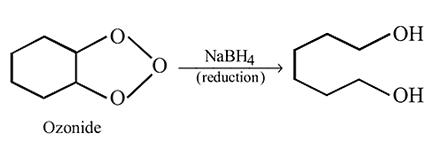
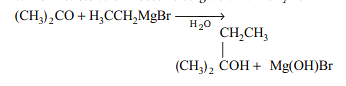
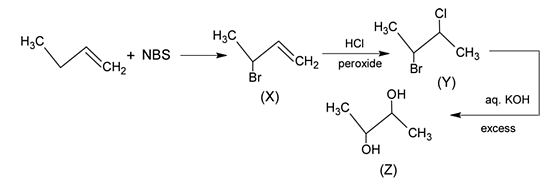
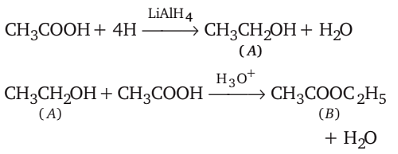
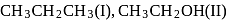 and
and 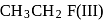 is in order
is in order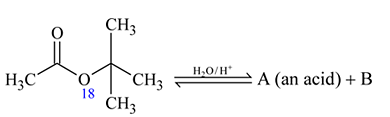
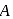 and
and 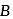 are, respectively,
are, respectively,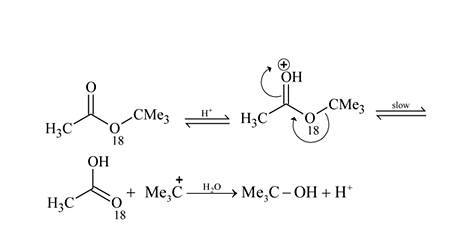
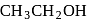 and
and