JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Preparation and Properties of Hydrogen - JEE MCQ
Test: Preparation and Properties of Hydrogen - JEE MCQ
Test Description
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Preparation and Properties of Hydrogen
Test: Preparation and Properties of Hydrogen for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Preparation and Properties of Hydrogen questions and answers have been
prepared according to the JEE exam syllabus.The Test: Preparation and Properties of Hydrogen MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Preparation and Properties of Hydrogen below.
Solutions of Test: Preparation and Properties of Hydrogen questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Preparation and Properties of Hydrogen solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Preparation and Properties of Hydrogen | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Preparation and Properties of Hydrogen - Question 1
During cracking of Natural gas, what is produced?
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 1
Test: Preparation and Properties of Hydrogen - Question 2
Which of the following is Radioactive?
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Preparation and Properties of Hydrogen - Question 3
What are the reactants and Lane's process?
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 3
Test: Preparation and Properties of Hydrogen - Question 4
Which of the following option is true regarding the boiling point of hydrogen isotopes?
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 4
Test: Preparation and Properties of Hydrogen - Question 5
Among the following, the correct statement is
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 5
Test: Preparation and Properties of Hydrogen - Question 6
Given below are two statements
Assertion (A): Protium and deuterium differ in their rates of reactions.
Reason (R): They have different enthalpies of bond dissociation.
The correct answer is
Assertion (A): Protium and deuterium differ in their rates of reactions.
Reason (R): They have different enthalpies of bond dissociation.
The correct answer is
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 6
Test: Preparation and Properties of Hydrogen - Question 7
The highly pure hydrogen is obtained when?
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 7
Test: Preparation and Properties of Hydrogen - Question 8
Which of the following holds true according to the properties of hydrogen?
I. Hydrogen is the lightest gas.
II. Hydrogen cannot be corrosive.
III. Deuterium is another name for heavy hydrogen.
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 8
Test: Preparation and Properties of Hydrogen - Question 9
Which of the following statements about hydrogen is incorrect?
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 9
Test: Preparation and Properties of Hydrogen - Question 10
Which one of the following pairs of substances on reaction will not evolve 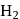 gas?
gas?
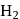 gas?
gas?
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 10
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 11
Test: Preparation and Properties of Hydrogen - Question 12
Hydrogen is evolved by the action of cold dil. 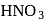 on
on
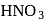 on
on
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 12
Test: Preparation and Properties of Hydrogen - Question 13
The property of hydrogen which distinguishes it from alkali metals is
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 13
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 14
Test: Preparation and Properties of Hydrogen - Question 15
Reactivity of hydrogen is compared to halogens.
Detailed Solution for Test: Preparation and Properties of Hydrogen - Question 15
|
352 videos|596 docs|309 tests
|
Information about Test: Preparation and Properties of Hydrogen Page
In this test you can find the Exam questions for Test: Preparation and Properties of Hydrogen solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Preparation and Properties of Hydrogen, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


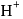 does not exist freely but as
does not exist freely but as 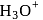 ion.
ion.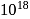 atoms of protium. Hydrogen is less reactive than halogen which are good oxidizing agents.
atoms of protium. Hydrogen is less reactive than halogen which are good oxidizing agents.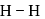 bond enthalpy
bond enthalpy 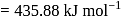
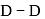 bond enthalpy
bond enthalpy 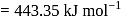 .
.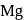 and
and 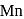 liberates
liberates 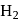 from dilute
from dilute 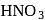 .
.














