Test: Properties of D-block elements - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Properties of D-block elements
Which of the following is not a condition for complex formation?
Which one of the following is a diamagnetic ion?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The inner transition elements are the elements in which the added electrons go to:
Which of the following pairs of ions are colourless?
Which of the following is not correct about transition metals?
Zinc and mercury do not show variable valency like  -block element because
-block element because
Which one of the following is diamagnetic ion?
Which group contains coloured ions out of
1. Cu2+
2. Ti4+
3. Co2+
4. Fe2+
A compound of a metal ion 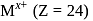 has a spin only magnetic moment of
has a spin only magnetic moment of  Bohr Magnetons. The number of unpaired electrons in the compound are
Bohr Magnetons. The number of unpaired electrons in the compound are
Cuprous ion is colourless while cupric ion is coloured because
 . This is due to its
. This is due to its
The correct statement about Cr2+ and Mn3+ among the following is (Given, atomic numbers of Cr = 24 and Mn = 25)
 in its +3 oxidation state?
in its +3 oxidation state?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|



 and
and  have
have  -shells completely filled hence they do not exhibit variable valency.
-shells completely filled hence they do not exhibit variable valency.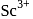 is diamagnetic as it does not contain any unpaired electron while others contain.
is diamagnetic as it does not contain any unpaired electron while others contain.
 are coloured ions
are coloured ions .
. where
where 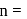 number of unpaired electrons
number of unpaired electrons 
 there is no unpaired electron,
there is no unpaired electron,  contains one unpaired electron hence coloured.
contains one unpaired electron hence coloured. is both paramagnetic and coloured.
is both paramagnetic and coloured.





 magnetic moment as 3.87
magnetic moment as 3.87 magnetic moment as 3.87
magnetic moment as 3.87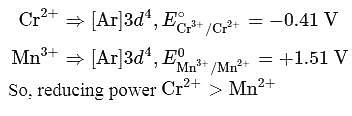
 has the electronic structure of
has the electronic structure of 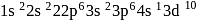 . One electron moves from the 45 -orbital to attain a more stable state with completely filled
. One electron moves from the 45 -orbital to attain a more stable state with completely filled  orbital.
orbital. will be
will be  .
.
 -orbital, and removing the electrons from the sub shell below it requires immense amounts of energy, this limits them to a +2 charge most of the time. Hence, the correct option is (c).
-orbital, and removing the electrons from the sub shell below it requires immense amounts of energy, this limits them to a +2 charge most of the time. Hence, the correct option is (c).














