JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - JEE MCQ
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - JEE MCQ
Test Description
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Refining & Reduction of Crude Metal, Flux and Refractory materials
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Refining & Reduction of Crude Metal, Flux and Refractory materials questions and answers have been
prepared according to the JEE exam syllabus.The Test: Refining & Reduction of Crude Metal, Flux and Refractory materials MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials below.
Solutions of Test: Refining & Reduction of Crude Metal, Flux and Refractory materials questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Refining & Reduction of Crude Metal, Flux and Refractory materials solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Refining & Reduction of Crude Metal, Flux and Refractory materials | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 1
Which of the following statements regarding electrolytic refining of copper is incorrect?
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 1
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 2
Which one of the following ores is concentrated by chemical leaching method?
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 3
If an impurity in a metal has a greater affinity for oxygen and is more easily oxidised than the metal, then the purification of metal may be carried out by
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 3
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 4
van Arkel method of purification of metals involves converting the metal to a
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 4
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 5
Which of the following pairs of metals is purified by van Arkel method?
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 5
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 6
Which of the following processes involve the roasting process?
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 6
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 7
In electrorefining of copper some gold is deposited as
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 7
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 8
In electro-refining of metal the impure metal is made the anode and a strip of pure metal, the cathode, during the electrolysis of an aqueous solution of a complex metal salt. This method cannot be used for refining of
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 8
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 9
Match the following items of List-I with those of List-II

The correct answer is
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 9
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 10
The metal purified by. Mond process is  and metal purified by van Arkel method is
and metal purified by van Arkel method is  . X and Y respectively are
. X and Y respectively are
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 10
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 11
During the electrolysis of cryolite, aluminium and fluorine are formed in ........ molar ratio :
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 11
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 12
Which method of purification is represented by the following equation?


Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 12
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 13
Refractory materials are generally used in furnaces because
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 13
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 14
Which one of the following ores is not concentrated by froth floatation process?
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 14
Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 15
Which one of the following is used as an acid flux in metallurgy?
Detailed Solution for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials - Question 15
|
352 videos|596 docs|309 tests
|
Information about Test: Refining & Reduction of Crude Metal, Flux and Refractory materials Page
In this test you can find the Exam questions for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Refining & Reduction of Crude Metal, Flux and Refractory materials, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


 (stable compound)
(stable compound) 

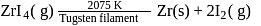




 Pure titanium
Pure titanium




 . Hence not concentrated by froth floatation process.
. Hence not concentrated by froth floatation process. (silica) is used as an acid flux in metallurgy. It reacts with gangue to form slag.
(silica) is used as an acid flux in metallurgy. It reacts with gangue to form slag.














