Test: Tin and lead compounds - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Tin and lead compounds
Ge(II)compounds are powerful reducing agents whereas Pb(IV) compounds are strong oxidants. It is because
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A metallic carbide on reaction with water gives a colourless gas which burns readily in air and gives a precipitate with ammonical silver nitrate solution. What is the gas evolved in reaction ?
Lead pipes are not suitable for drinking water because
Identify the incorrect statements from the following.
I. Tin in +2 state acts as reducing agent while lead in +4 state acts as strong oxidising agent.
II. Silicon exists as both 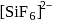 and
and  forms.
forms.
III. The hybridisation of carbon in fullerene is s3.
IV. Among Ge,Sn and Pb lowest melting point is for Sn.
A solid element (symbol Y) conducts electricity and forms two chlorides YCln (colourless volatile liquid) and YCln−2 (a colourless solid). To which one of the following groups of the periodic table does Y belong?
Which of the following conceivable structures for CCl4 will have a zero dipole moment?
To a piece of charcoal, sulphuric acid is added. Then:
With respect to graphite and diamond, which of the statement (s) given below is (are) correct?
1. Graphite has higher electrical conductivity than diamond
2. Graphite is harder than diamond
3. Graphite has higher C - C bond order than diamond
4. Graphite has higher thermal conductivity than diamond
The correct order of increasing C-O bond length of CO, CO2 and  is:
is:
Which of the following statement(s) is / are incorrect for CO2?
(i) In laboratory CO2 is prepared by the action of dilute HCl on calcium carbonate
(ii) Carbon dioxide is a poisonous gas
(iii) Increase in carbon dioxide content in atmosphere lead to increase in green house effect.
(iv) CO2 as dry ice is used as a refrigerant for ice cream and frozen food.
Certain organic compound on combustion produces three gaseous oxides A, B and C. A and C turned lime water milky, B turned anhydrous CuSO4 blue and C turned K2Cr2O7 solution green. The elements present in organic compounds are
The ions present in Al4C3,CaC2 and Mg2C3 are respectively
Consider the following statements:
I. In diamond, each carbon atom is sp3-hybridised.
II. Graphite has planar hexagonal layers of carbon atoms.
III. Silicones being surrounded by non-polar alkyl groups are water repelling in nature
IV. The order of catenation in group 14 elements is Si > C > Sn > Ge > Pb.
The correct statements are
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|




 paramagnetic, coloured gas
paramagnetic, coloured gas
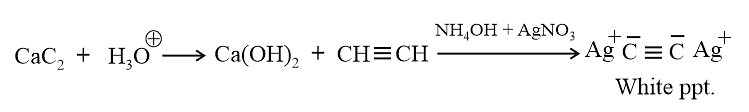

 is known but
is known but 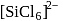 does not exist, this is because due to larger size of Cl atom as compared to F atom it is not possible to accommodate six atoms of Cl around Si atom. (III). Fullerens structure have carbon atoms both in sp2 and sp3 hybridised system.
does not exist, this is because due to larger size of Cl atom as compared to F atom it is not possible to accommodate six atoms of Cl around Si atom. (III). Fullerens structure have carbon atoms both in sp2 and sp3 hybridised system.

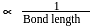 Hence, the decreasing
Hence, the decreasing 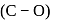 bond length is:
bond length is:
 are present in Al4C3,CaC2 and Mg2C3 are respectively
are present in Al4C3,CaC2 and Mg2C3 are respectively














