Test: Valence Bond Theory, Effective Atomic Number & Magnetic Properties - NEET MCQ
25 Questions MCQ Test Chemistry Class 12 - Test: Valence Bond Theory, Effective Atomic Number & Magnetic Properties
Only One Option Correct Type
Direction (Q. Nos. 1- 10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which one of the following is wrongly matched?
In which of the following the central atom has sp3d2-hybridisation?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which is the diamagnetic?
A magnetic moment of 1.73 BM will be shown by one among following.
Which one of the following complex species does not involve inner orbital hybridisation?
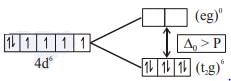
Which of the following complex has zero magnetic moment (spin only)?
The number of unpaired electrons calculated in [Co(NH3)6]3+ and [CoF6]3- are
The tetrachloro complexes of Ni(ll) and Pd(ll) respectively are (atomic number of Ni and Pd are 28 and 46 respectively).
Which one of the following is an inner orbital complex as well as diamagnetic in behaviour? (Atomic number, Zn = 30, Cr= 24, Co = 27, Ni = 28)
The pair of compounds having the same hybridisation for the central atom is
One or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
The formation of complex which involves d-orbitals of outer shell are called
sp3-hybridisation is found in
Regarding the complex [Co(H2O)6]2- , correct statement(s) is/are
For which the EAN value is equal to the atomic number of a noble gas?
[Fe(H2O)6]2+ and [Fe(CN)6]4- differ in
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
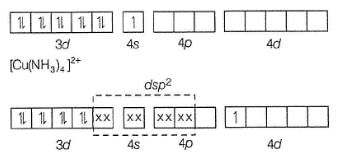
When crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN- ions but give test for new ion [Fe(CN)6]4- called ferrocyanide ion.
Q.
The hybridisation and geometry and magnetic property of [Cu(NH3)4]2+ ion are
When crystals of CuSO4 . 4NH3 are dissolved in water, there is hardly any evidence for the presence of Cu2+ ions or ammonia molecules. A new ion [Cu(NH3)4]2+ is furnished in which ammonia molecules are directly linked with the metal ion. Similarly, aqueous solution of Fe(CN)2 .4KCNdoes not give tests of Fe2+ and CN- ions but give test for new ion [Fe(CN)6]4- called ferrocyanide ion.
Q.
The hybridisation, geometry and magnetic property of [Fe(CN)6]4- are
Matching List Type
Direction (Q. Nos. 18 and 19) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d) out of which one is correct.
Q.
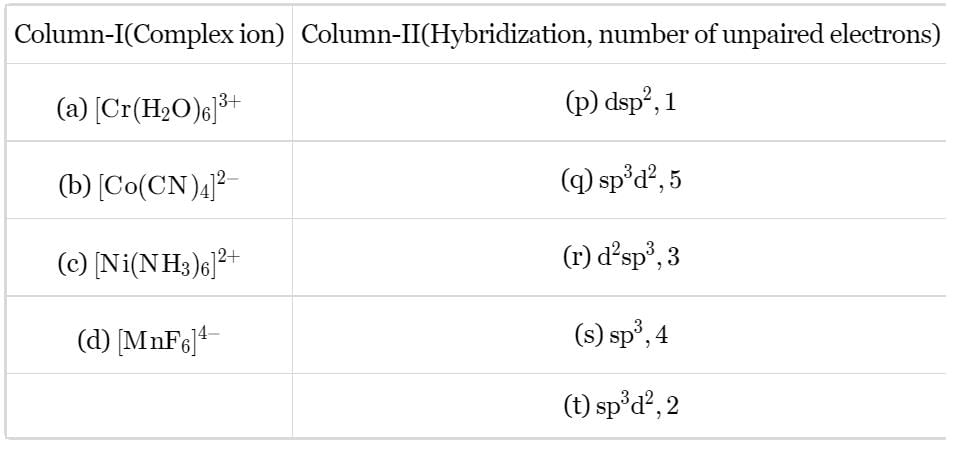
Match the Column I with Column II and mark the correct option from the codes given bleow.
Match the Column I with Column il and mark the correct option from the codes given bleow.
One Integer Value Correct Type
Direction (Q. Nos. 20-24) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
How many of the following species obey Sidgwick EAN rule?
K3[Fe(CN)6], [Ru(CO)5], [Cr(NH3)6] 3+, [Co(NH3)6]3+, [Ni(NH3)6]2+, [Fe(CO)5],[W(CO)6]
How many of the following are diamagnetic?
[Zn(OH)4]2-,[Ni(NH3)6]2+, K4[Fe(CN)6], K3[Fe(CN)6], [Cu(NH3)4]2+,[PdBr4]2-, [Ni(CO)4], [CoF6] 3-
The number of unpaired electrons in the complex [Mn(acac)3] is
(Atomic number of Mn= 25)
The magnetic moment of [Ru(H2O)6]2+ corresponds to the presence of ...... unpaired electrons.
The number of unpaired electrons in [Fe(dipy)3]2+ are
Statement Type
Direction (Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Both [Ni(CN)4]2- and [NiCI4]2- have same shape and same magnetic behaviour.
Statement II : Both are square planar and diamagnetic
|
108 videos|286 docs|123 tests
|