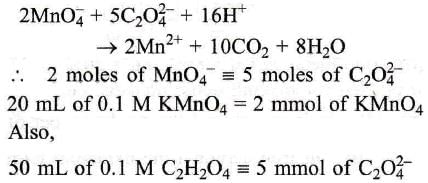WBJEE Chemistry Test - 1 - JEE MCQ
30 Questions MCQ Test WBJEE Sample Papers, Section Wise & Full Mock Tests 2025 - WBJEE Chemistry Test - 1
KMnO₄ reacts with oxalic acid according to the equation :
2MnO₄ + 5C₂ O₄²⁻ + 16H⁺ →2MN² + 10CO₂ + 8H₂O Here 20 ml. of 0.1 M KMnO₄ is equivalent to ?
2MnO₄ + 5C₂ O₄²⁻ + 16H⁺ →2MN² + 10CO₂ + 8H₂O Here 20 ml. of 0.1 M KMnO₄ is equivalent to ?
The compound 'A' when treated with methyl alcohol and few drops of H₂SO₄ give wintergreen smell. The compound 'A' is
The product acetal is produced by reacting alcohol in the presence of dry HCl with
In the borax bead test of Co²⁺, the blue colour of bead is due to the formation of
The heats of combustion of carbon and carbon monoxide are −393.5 and −283.5 kJ mol −1, respectively. The heat of formation (in kJ) of carbon monoxide per mole is:
The rate constant for the first and zero reactions in terms of molarity, M units respectively
Latent heat of vaporisation of a liquid at 500 K and 1 atm pressure is 10.0 kcal/mol. What will be the change in internal energy (ΔE) of 3 mol of liquid at same temperature?
Among the following, the species having square planar geometry for central atom are
1. XeF4
2. SF4
3. [NiCl4]2−
4. [PtCl4]2−
16 gm of oxygen and 3 gm of hydrogen are mixed and kept in 760 mm pressure at 0ºC. The total volume occupied by the mixture will be nearly
|
3 videos|10 docs|54 tests
|
|
3 videos|10 docs|54 tests
|