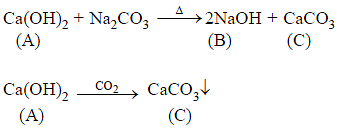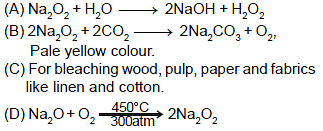s-Block Elements (Alkali and Alkaline Earth Metals) - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - s-Block Elements (Alkali and Alkaline Earth Metals) - 1
An alkaline earth metal (M) gives a salt with chlorine, which is soluble in water at room temperature. It also forms an insoluble sulphate whose mixture with a sulphide of a transition metal is called `lithopone'-a white pigment. Metal M is-
The hydroxide of alkaline earth metal, which has the lowest value of solubility product (Ksp) at normal temperature (25°C) is-
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following carbonate of alkali metals has the least thermal stability ?
The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals, the lowest solubility of LiF in water is due to
The formula of soda ash is
The golden yellow colour associated with NaCl to Bunsen flame can be explained on the basis of -
The order of solubility of lithium halides in non polar solvents follows the order.
The correct order of solubility is -?
When K2O is added to water, the solution becomes basic in nature because it contains a significant concentration of -
Which of the following is incorrect for sodium peroxide ?
A metal [X] on heating in nitrogen gas gives [Y].[Y] on treatment with H2O gives a colourless gas which when passed through CuSO4 solution gives a blue colour. [Y] is
Which is not correctly matched ?
(1) Basic strength of oxides Cs2O< />2O< K2O < Na2O < Li2O(2) Stability of peroxides Na2O2 < K2O2 < Rb2O2 < Cs2O2
(3) Stability of bicarbonates LiHCO3 < NaHCO3 < KHCO3 < RbHCO3 < CsHCO3
(4) Melting point NaF < NaCl < NaBr < NaI
The set representing the correct order of first ionization potential is
The ionization enthalpies of the alkali metals decrease down the group from Li to Cs because :
Which of the follownig acids will not evolve H2 gas on reaction with alkali metals
The first four ionization potentials (eV) are given for two elements X and Y. Identify them.
X : 8.296, 25.149, 37.92, 259.298
Y : 5.318, 47.29, 71.65, 98.88
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|