Test: Surface Chemistry - 1 (Old NCERT) - NEET MCQ
25 Questions MCQ Test Chemistry Class 12 - Test: Surface Chemistry - 1 (Old NCERT)
Which among the following is a property of physisorption?
Powdered substances are more effective adsorbents than their crystalline forms because:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which among the following is true for chemisorptions?
The extent of adsorption increases with the ______.
The number of phases in colloidal system are:
Which among the following is not the method of colloidal purification?
Chromatography is based on the principle of
Which among the following substances is an example of multimolecular colloids?
Tyndall effect in colloidal solution is due to:
Adsorption isobar is a curve showing variation of adsorption ______.
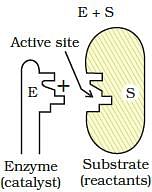
Which step of enzyme catalysis mechanism is shown in figure?
Which of the following statement is true about Langmuir’s adsorption?
When adsorption of oxalic acid is carried out on activated charcoal, then activated charcoal is known as
Which of the following metal solution cannot be prepared by Bredig’s arc method?
The average molecular mass of colloidal can be determined by
Which of the following processes does not involve a catalyst?
Which type of a property is the Brownian movement of colloidal solution?
Which among the following is adsorbed greatly by activated charcoal?
Which among the following is an example of sorption?
The adsorbent used in decolouration of vinegar and sugar solution is
Which of the following kinds of catalysis can be explained by the adsorption theory?
|
100 videos|282 docs|123 tests
|












