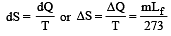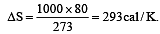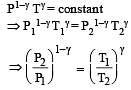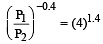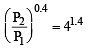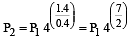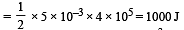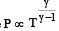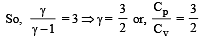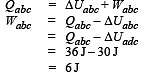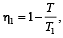31 Year NEET Previous Year Questions: Thermodynamics - 1 - NEET MCQ
14 Questions MCQ Test Physics Class 11 - 31 Year NEET Previous Year Questions: Thermodynamics - 1
In thermodynamic processes which of the following statements is not true? [2009]
The internal energy change in a system that has absorbed 2 kcals of heat and done 500 J of work is: [2009]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If ΔU and ΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true? [2010]
During an isothermal expansion, a confined ideal gas does –150 J of work against its surroundings. This implies that [2011]
When 1 kg of ice at 0°C melts to water at 0°C, the resulting change in its entropy, taking latent heat of ice to be 80 cal/°C, is [2011]
A mass of diatomic gas (γ= 1.4) at a pressure of 2 atmospheres is compressed adiabatically so that its temperature rises from 27°C to 927°C.The pressure of the gas in final state is [2011M]
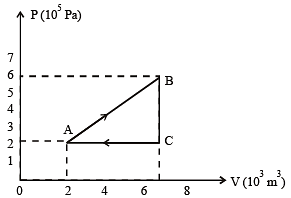
A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is : [2012]

One mole of an ideal gas goes from an initial state A to final state B via two processes : It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is : [2012]
If Q1, Q2, Q3 indicate the heat a absorbed by the gas along the three processes and ΔU1, ΔU2, ΔU3 indicate the change in internal energy along the three processes respectively, then
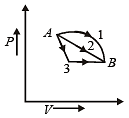
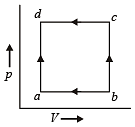
A gas is taken through the cycle A → B → C → A, as shown in figure. What is the net work done by the gas ? [NEET 2013]
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of 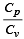 for the gas is [NEET 2013]
for the gas is [NEET 2013]
A system is taken from state a to state c by two paths adc and abc as shown in the figure. The internal energy at a is Ua = 10 J. Along the path adc the amount of heat absorbed δQ1 = 50 J and the work done δW1 = 20 J whereas along the path abc the heat absorbed δQ2 = 36 J. The amount of work done along the path abc is [NEET Kar. 2013]
Which of the following relations does not give the equation of an adiabatic process, where terms have their usual meaning? [NEET Kar. 2013]
Two Carnot engines A and B are operated in series. The engine A receives heat from the source at temperature T1 and rejects the heat to the sink at temperature T. The second engine B receives the heat at temperature T and rejects to its sink at temperature T2. For what value of T the efficiencies of the two engines are equal? [NEET Kar. 2013]
|
97 videos|378 docs|103 tests
|